An enzyme involved in cancer and aging gets a close-up
Understanding what telomerase looks like could guide therapies for cancer, other illnesses
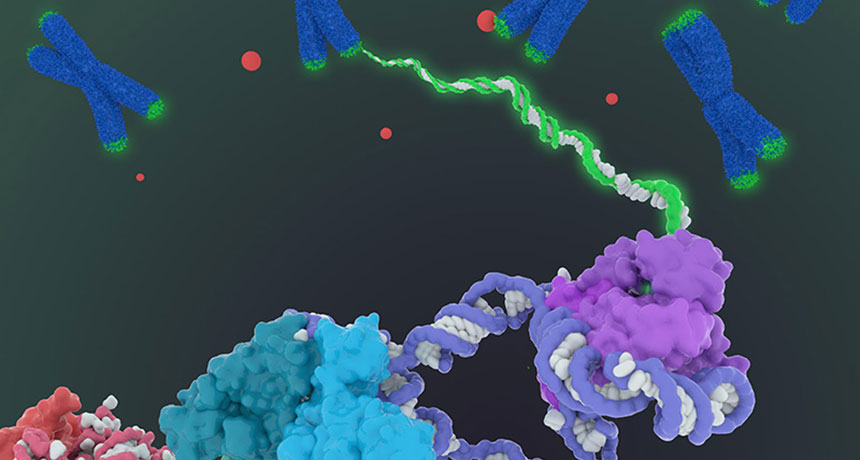
HANDY ADD Telomerase (bottom structure), a complex of proteins and RNA, adds DNA to telomeres (green caps), the ends of chromosomes (blue X’s). The telomeres shorten when cells divide.
Janet Iwasa







