LIFE'S CYCLES Throughout the human body, circadian clocks keep activities running on a daily schedule of day and night. New work is shedding light on the origin and evolution of biological clocks.
Todd Churin
The Earth has rhythm. Every 24 hours, the planet pirouettes on its axis, bathing its surface alternately in sunlight and darkness.
Organisms from algae to people have evolved to keep time with the planet’s light/dark beat. They do so using the world’s most important timekeepers: daily, or circadian, clocks that allow organisms to schedule their days so as not to be caught off guard by sunrise and sunset.
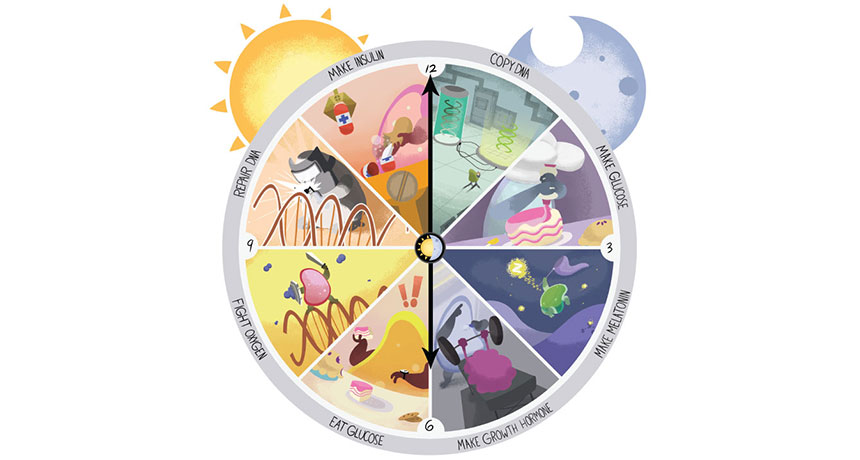
A master clock in the human brain appears to synchronize sleep and wake with light. But there are more. Circadian clocks tick in nearly every cell in the body. “There’s a clock in the liver. There’s a clock in the adipose [fat] tissue. There’s a clock in the spleen,” says Barbara Helm, a chronobiologist at the University of Glasgow in Scotland. Those clocks set sleep patterns and meal times. They govern the flow of hormones and regulate the body’s response to sugar and many other important biological processes (SN: 4/10/10, p. 22).
Having timekeepers offers such an evolutionary advantage that species have developed them again and again throughout history, many scientists say. But as common and important as circadian clocks have become, exactly why such timepieces arose in the first place has been a deep and abiding mystery.
Many scientists favor the view that multiple organisms independently evolved their own circadian clocks, each reinventing its own wheel. Creatures probably did this to protect their fragile DNA from the sun’s damaging ultraviolet rays. But a small group of researchers think otherwise. They say there had to be one mother clock from which all others came. That clock evolved to shield the cell from oxygen damage or perhaps provide other, unknown advantages.
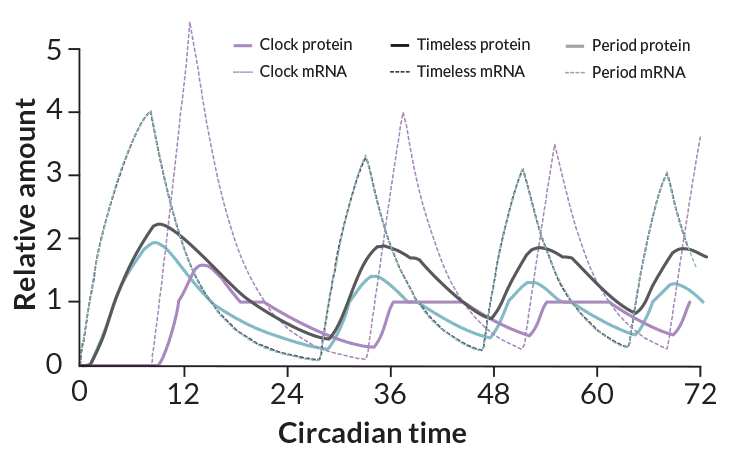
Circadian clocks don’t have gears and hands. They’re composed of RNA molecules and proteins that oscillate in abundance. At particular times of day, certain clock proteins switch on production of messenger RNA, used by the cell to bake fresh batches of other clock proteins. Eventually levels of those proteins reach a certain threshold; they then shut off creation of the messenger RNA that produces them. The self-suppressing proteins disintegrate or get nibbled away by other proteins until their levels fall below a threshold, signaling the need for another batch, and the cycle starts again.
Just as Rolex, Timex, Swatch and Seiko make their own versions of a wristwatch, organisms including cyanobacteria, fungi, plants and insects have all invented their own varieties of circadian clocks. The cycling proteins are as different among these organisms as digital watches are from precision quartz clockworks. But all of them mark days with the predictable ebb and flow of messenger RNA and protein production.
There’s no doubt that today’s circadian clocks are must-have accessories for most organisms living on Earth’s surface. But does the run-away-from-the-light origin story make sense?
A main piece of evidence in favor of the “flight from light” idea is that cells tend to replicate their DNA at night safely under cover of darkness and repair it during the day as damage from UV light accumulates. Some of the same protein cogs that drive the circadian clocks are also involved in DNA repair, further solidifying the connection.
“That’s a nice idea,” says circadian cell biologist John O’Neill of the MRC Laboratory of Molecular Biology in Cambridge, England, “but it doesn’t fit with modern data.”
Going way back
Several lines of evidence argue against flight from light as the common force propelling the evolution of circadian clocks, says O’Neill, one of the scientists rewriting the circadian clock origin story.
If the cycle arose to protect DNA, one would expect cycling to happen only if there was DNA to protect. But circadian rhythms can happen in a test tube without DNA.
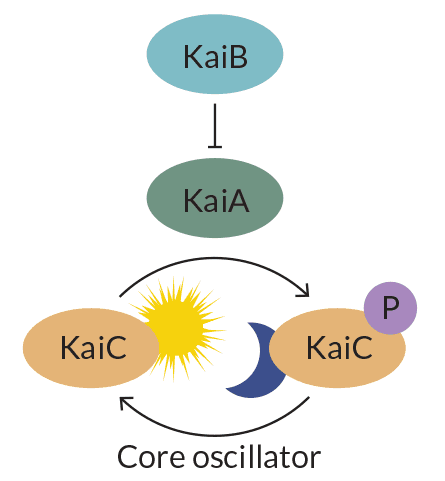
A type of cyanobacteria, or blue-green algae, known as Synechococcus elongatus has one of the simplest known circadian clocks. It consists of three proteins called KaiA, KaiB and KaiC. Those three gears, along with two accessory proteins, help the algae prepare for sunrise by stockpiling proteins needed for photosynthesis and other important daily activities.
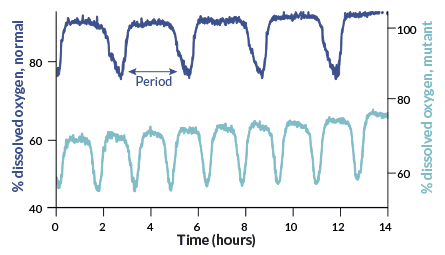
Plop the three clock proteins into a test tube. Add energy from adenosine triphosphate (better known as ATP), and the clock will rhythmically add and subtract phosphate molecules from KaiC, Takao Kondo of Nagoya University in Japan and colleagues reported in Science in 2005. The finding shook up circadian researchers because it showed that clocks can operate without DNA. It also revealed that they don’t need to switch messenger RNA and protein production on and off to keep time.
Those blue-green algae and the mysterious, unnamed ancestor of insects and animals formed different branches on the evolutionary tree more than 1 billion years ago. Clock proteins of S. elongatus are nothing like the central timekeeping proteins of mammals. So some researchers doubted that DNA-free clocks existed in organisms more complex than algae.
O’Neill and his collaborator Akhilesh Reddy of the University of Cambridge thought that they could find DNA-free clocks elsewhere. They decided to look for circadian clocks in human red blood cells, which lack a nucleus where DNA is stored. Without DNA, there is also no messenger RNA production, which is essential for the classic circadian clocks to work. Nevertheless, the cells still have circadian rhythms, O’Neill and Reddy reported in Nature in 2011.
The red blood cell clock is entirely different from the protein and messenger RNA cycle that synchronizes nucleus-containing cells with the sun. In the red blood cells, antioxidant proteins called peroxiredoxins accept or give up oxygen molecules in a persistent circadian rhythm. Their action helps mop up hydrogen peroxide, a by-product of a cell’s normal energy-manufacturing activities. Hydrogen peroxide and other oxidants can damage many components of a cell, so keeping them in check is essential for survival.
Peroxiredoxins are found in a wide variety of organisms, including marine algae called Ostreococcus tauri. Working with other collaborators, O’Neill and Reddy examined the peroxiredoxins in the algae. “Just like the red blood cells, there was a rhythm,” O’Neill says. The amount of oxygen molecules clinging to the peroxiredoxins rose and fell in a persistent 24-hour cycle. The team reported that finding in the same 2011 issue of Nature.
A year later, the researchers reported in Nature that they had found peroxiredoxin cycles in fruit flies, the plant Arabidopsis thaliana, a fungus called Neurospora crassa, S. elongatus cyanobacteria and an archaean called Halobacterium salinarum. Together, the organisms represent all of the major domains of life. If every domain has peroxiredoxin clocks, the antioxidants are most likely ancient, probably dating back billions of years.
The oxygen menace
No one knows for sure how far back those antioxidant clocks go, but O’Neill has a time frame in mind: 2.5 billion years. That’s when cyanobacteria, which had recently begun using photosynthesis to fuel their activities, started releasing vast amounts of oxygen in the Great Oxidation Event. While photosynthesis and an oxygen-rich atmosphere are now considered necessities, oxygen was poison for Precambrian life-forms. Organisms that could not tolerate free oxygen either died or ended up in the anaerobic deep sea. “If they didn’t die off, they had to cope,” O’Neill says.
Oxygen would have been a problem mainly during the day, when photosynthesis was taking place. Organisms that geared up their antioxidant defenses — stripping peroxiredoxin of oxygen molecules so that it could sponge up hydrogen peroxide when the sun peeked over the horizon — would get a jump on survival. A timing mechanism that could anticipate oxygen’s arrival instead of just reacting to it would be “such an enormous advantage,” says O’Neill, “that it just became hardwired.”
Story continues below graphic
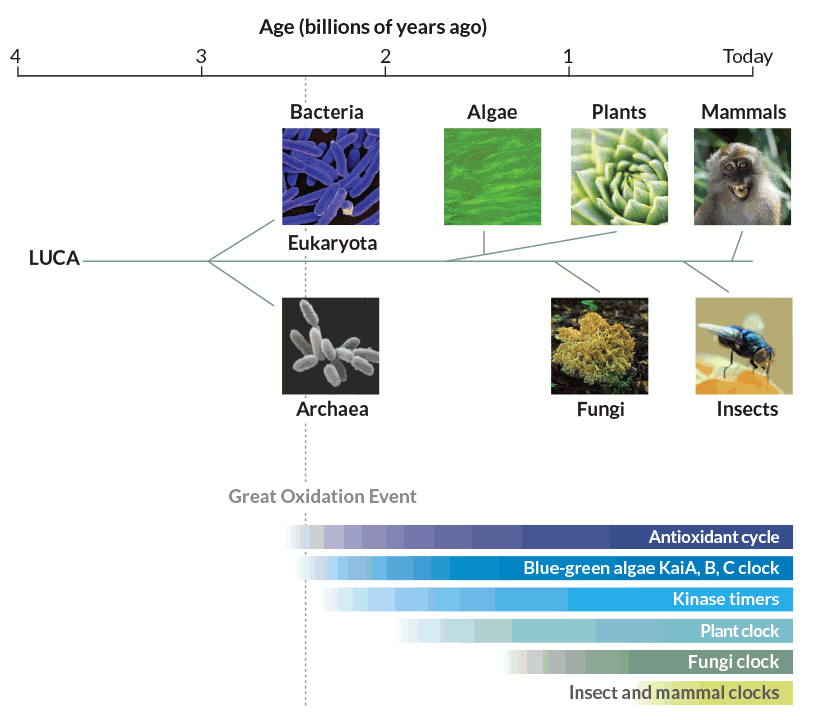
Peroxiredoxins themselves are not clock gears. They are more like a clock’s hands; the amount of oxygen bound to them is an indicator of time being kept by an as yet unknown and even more ancient central timekeeper. That mysterious clock was such an advantage that organisms have maintained it across evolutionary history, tinkering with it as needed. Like watches that can tell time in several time zones and display a.m. and p.m. plus calendar information, circadian clocks have added components to keep track of different environmental challenges, O’Neill speculates.
Other researchers have proposed that because the circadian clock proteins of cyanobacteria, animals and plants are so different, ancestors of those organisms must have evolved clocks independently. But even though the core cogs are different, says O’Neill, “you always find the same handful of kinases that set the speed of the clock.”
Kinases are proteins that tack phosphate molecules onto other proteins, slating them for destruction or altering their function. Two of the most important of those kinases — casein kinase 1 (CK1) and glycogen synthase kinase 3 (GSK3) — are also important in pacing peroxiredoxin clocks, O’Neill has found. They may be the ancestral clocks he and others have been seeking.
Even organisms that don’t have circadian rhythms have kinase-driven peroxiredoxin cycles, O’Neill, Helen Causton of Columbia University and colleagues reported in the April 20 Current Biology. Baker’s yeast, Saccharomyces cerevisae, has neither proteins recognizable as clock proteins nor a 24-hour cycle. That doesn’t mean yeast can’t keep time. They have about eight three-hour-long respiratory oscillations in which their rate of oxygen consumption rises and falls. Chemically blocking yeast’s version of CK1 slows down the yeast oscillation. Messing with CK1 also alters the circadian rhythm in mouse cells, the researchers reported.
Those findings suggest that kinases are important for establishing the rhythm of circadian timers. The researchers think the kinases may have formed a simple timer, similar to cyanobacteria’s KaiA, B, C system. With those simple gears in place, organisms would have added more gears to form the clocks we see today, O’Neill says. There is still no evidence, however, that the kinases are the ancestral molecules that spawned today’s clocks.
O’Neill admits that there is another possibility. There may be no mother clock. Cell biology may simply be driven by biochemical reactions that naturally fall into rhythmic patterns. “I don’t like that possibility because it’s quite difficult to disprove” or test, he says. The only real way to demonstrate that’s not the answer is to go back and find the master clock. But, O’Neill laments, “the problem with all these evolutionary arguments is you can’t test them without a time machine.”
Independent evolution
Not everyone is enamored with the peroxiredoxin hypothesis. “They have a very grandiose scheme,” says Joseph Takahashi, a circadian geneticist and neuroscientist at the University of Texas Southwestern Medical Center in Dallas. “There’s just no evidence.”
True enough, acknowledges O’Neill. “We don’t have a mechanism.” All they have are observations that are incompatible with classic models that describe clocks as machines of oscillating proteins and messenger RNA that evolved as light-avoidance mechanisms.
Central to O’Neill’s argument is the idea that there must have been an ancestral clock upon which all clock-carrying organisms built their daily timers. Other researchers aren’t so quick to dismiss independent evolution.
“I don’t think we should assume it’s hard to build a clock,” says Susan Golden, a microbiologist at the University of California, San Diego. The timing mechanisms seen in nature today are just the ones that have stuck around. Organisms may have tried and rejected other timers or other rhythms. Recently, independent research groups found that a marine worm has a lunar clock, and a sea louse has a tidal timer (SN: 11/2/13, p. 6). Golden’s lab group is tinkering with a cyanobacterial circadian clock to see if it can keep time on a different scale — weeks or hours instead of days, for instance.
Real-world advantages
Although no one has dug up the original clock, some scientists are philosophizing about why such a mechanism might have been useful in the first place. Avoiding oxygen toxicity and fleeing damaging light aren’t the only reasons a circadian clock is a good idea. Some researchers say the advantage of having a clock may be in keeping contradictory chemical reactions separated or making cells run more smoothly by creating a production schedule for molecules needed for each step of a biochemical chain reaction.
“We wonder why is the clock turning on and off metabolism each day rather than just letting everything run with the taps on,” Takahashi says. He and colleagues are testing the idea that producing things in big bursts the way the clock dictates is more energy-efficient than making small amounts over a longer period. One 2010 computer simulation estimates that circadian clocks may save organisms enough energy to grow 15 percent faster. Measuring that possible advantage in the real world, however, has been difficult.
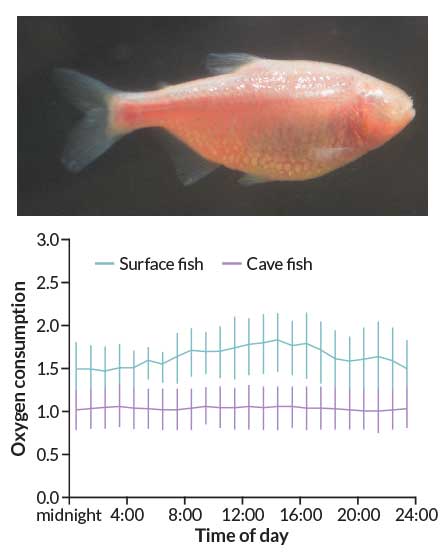
Moran put the surface and cave fish in swimming tubes and flowed water over them so that they swam at “a slow walk” for several days. He measured how much oxygen the fish used. As expected, the surface fish used more oxygen during the day than at night. But the cave fish used the same amount of oxygen day and night. “Maybe it’s just that one fish,” he recalls thinking. “So then we put the next fish in.” That fish’s oxygen consumption stayed flat, too.
By keeping their metabolism at a steady rate all day, rather than a rhythmic boost that follows light cycles, the cave fish saved 27 percent of their energy, the team reported last September in PLOS ONE. When both surface and cave fish were tested in darkness, cave fish did even better, expending 38 percent less energy than surface fish did.
The finding doesn’t mean Takahashi is wrong about circadian clocks saving energy in a rhythmic world. It’s just that cave fish live in a fairly constant — dark — environment. In that case, wonders Moran, “what are you rousing your metabolism for?” If fish gear up in anticipation of an event, “and it doesn’t happen, what a waste,” he says. But in a world where sunrise is the gold standard of predictability, circadian clocks may indeed be thrifty options.
Just because some animals in extreme environments have radically different clocks doesn’t mean that living without rhythm is a good idea for everybody. “I’m rather skeptical that — except in some freaky situations — life is better without a clock,” says Helm, the chronobiologist from Glasgow. Cave fish also lack eyes, but nobody would argue that means eyes are unimportant, she says.
Maybe, says Golden, clocks didn’t evolve for just one reason. Clocks, she says, may generally be necessary for “not being jerked around by the environment.”
This story appears in the July 25, 2015 issue with the headline, “Life’s cycles: Poking holes in classic models of circadian clock evolution.”
Editor’s note: This story was updated on July 16, 2015, to amend the caption of the “Mother of all clocks” infographic. It previously incorrectly stated that the last universal common ancestor of all living things may have had a primitive circadian clock.