Newborns’ weak immunity may allow helpful bacteria to gain a foothold
Though infant immune systems raise risk of infection, they also allow good microbes into the body
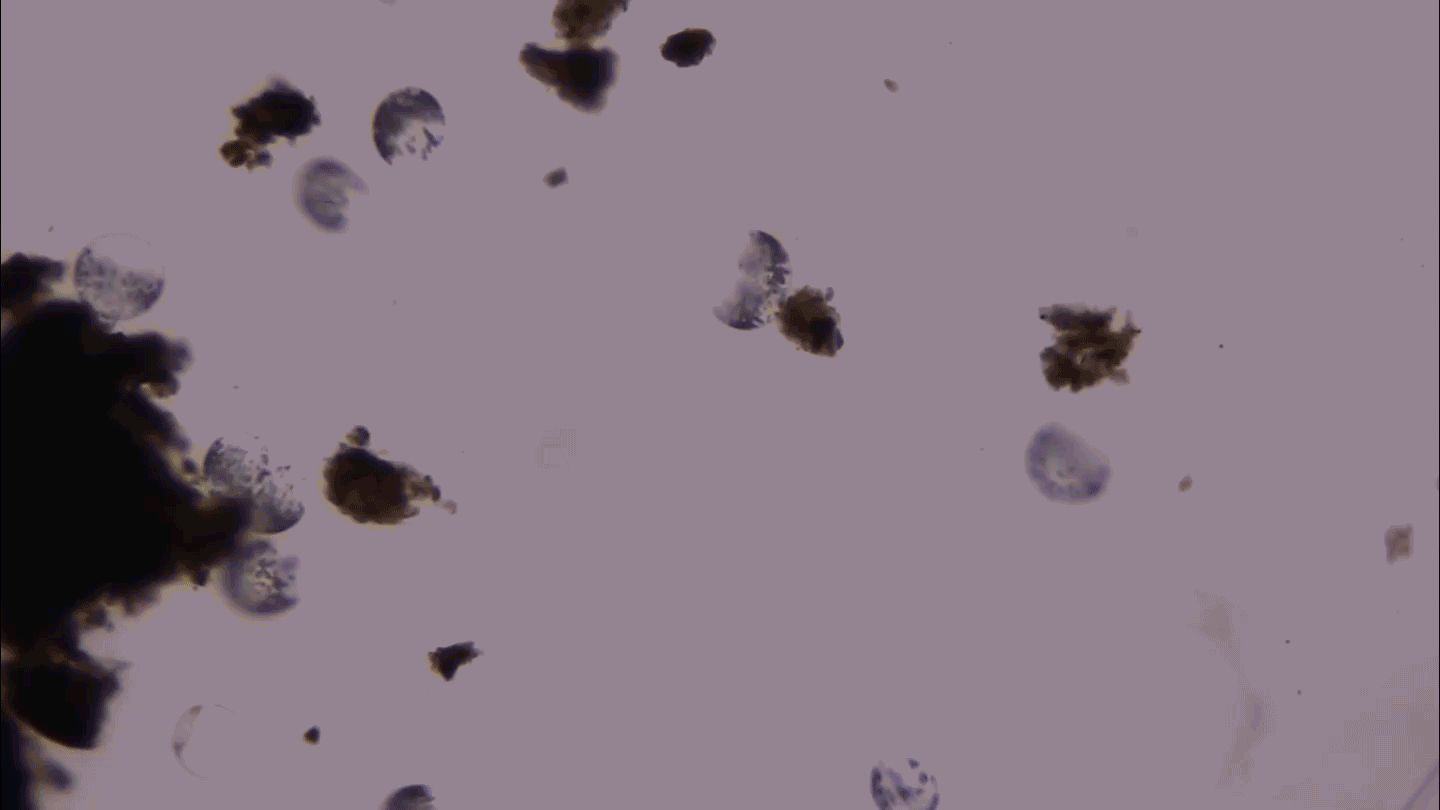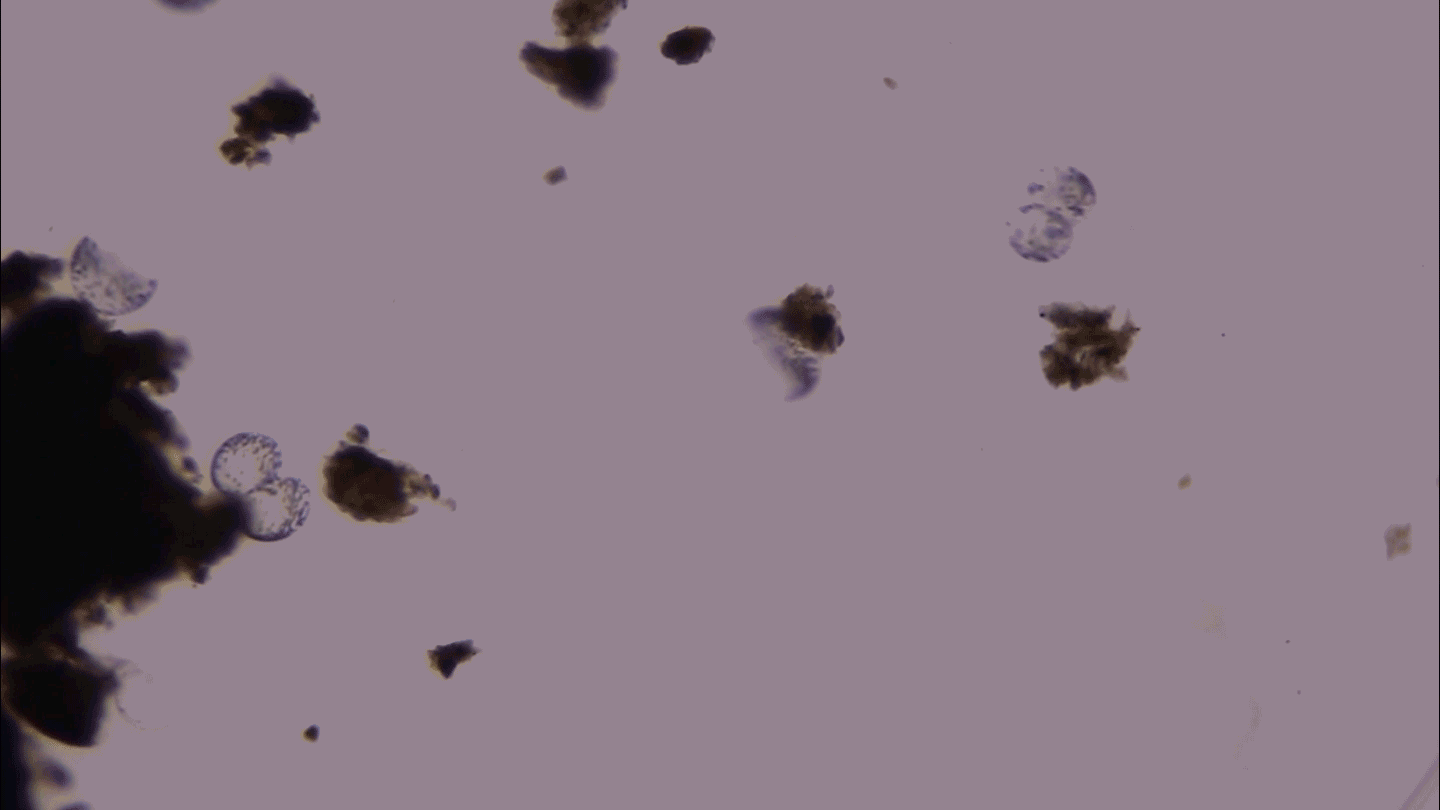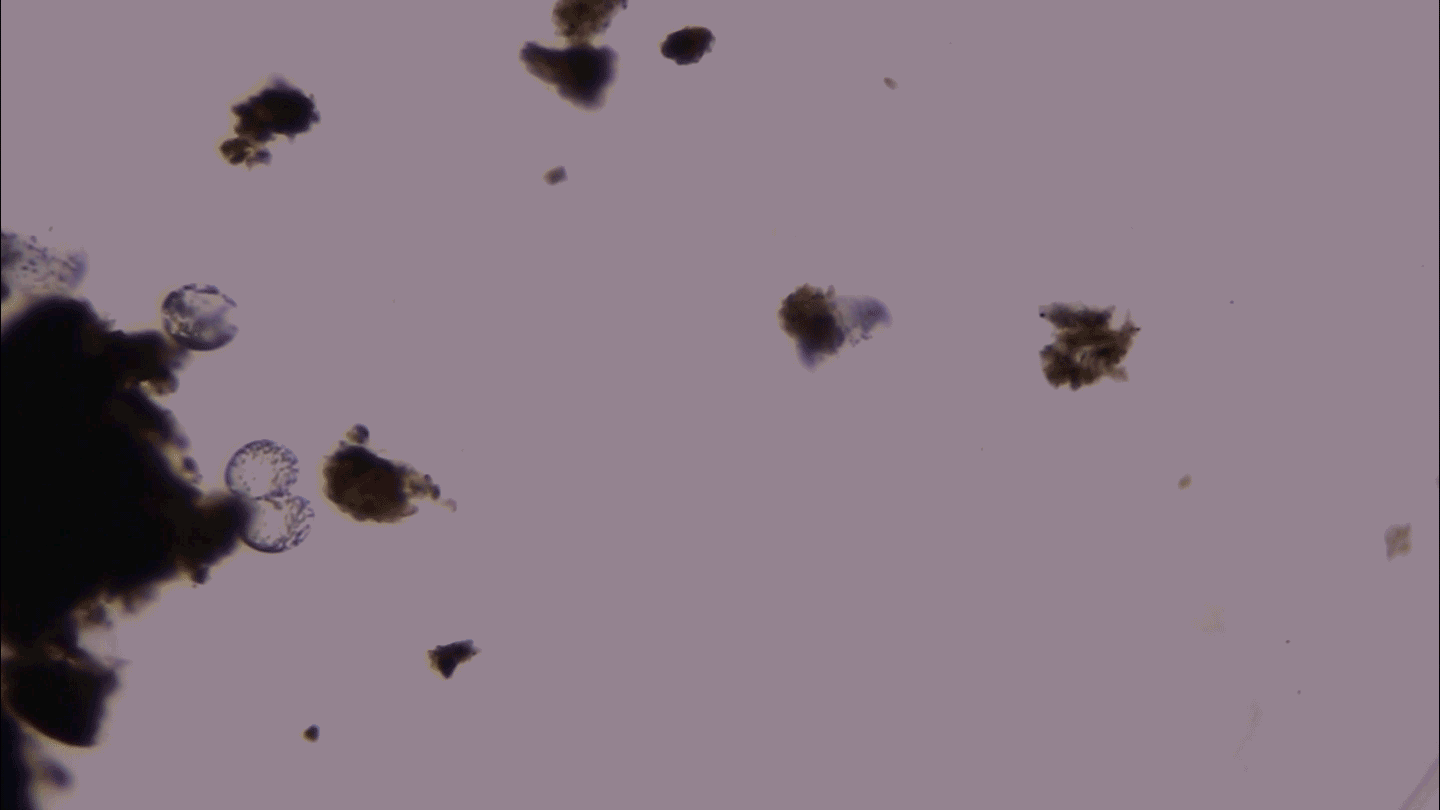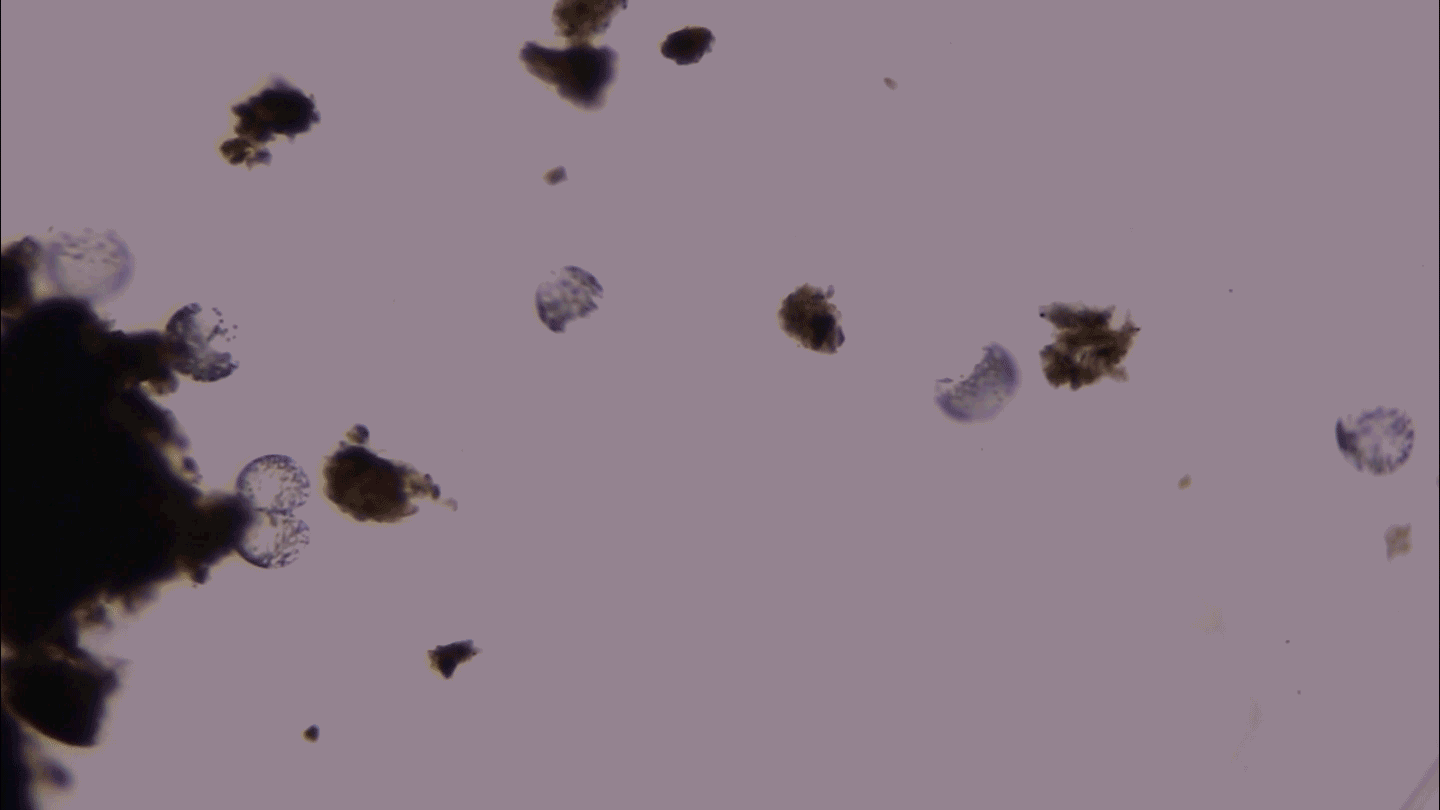
GROWING GOOD BACTERIA An immature form of red blood cell helps suppress the immune system of newborns, experiments with mice suggest. Immune suppression leaves infant mice and humans susceptible to infection but may allow good bacteria to settle in the gut.
KarenMower/iStockphoto
The seeming failure of newborns to muster a robust defense against infections is a trade-off that delivers long-term benefits, a new study suggests. In infants, the body’s immune army stands down for a month or two and then gears up. While this gap leaves babies at risk of infection, it also may allow beneficial bacteria to populate an infant’s intestines, a development that carries lifelong advantages, researchers working with mice report November 6 in Nature.
The findings suggest that the lackluster response of the neonatal immune system “is a normal developmental feature,” says biochemist Sidney Morris Jr. of the University of Pittsburgh School of Medicine, who wasn’t involved in the study. The immune suppression shows up “during the transition from the sterile in utero setting to a decidedly nonsterile external environment,” he says.
The human body houses billions of helpful microbes, called commensal bacteria, which aid in digestion and provide other services, says study coauthor Sing Sing Way, an infectious disease pediatrician at Cincinnati Children’s Hospital Medical Center. “They occupy a niche that prevents more-pathogenic bacteria from occupying the intestines,” he says. “Commensal bacteria are a kind of protective barrier.”
In newborn humans and mice, these microbes need to get ensconced in the intestines without the immune system spotting them and calling in the troops.
In neonatal mice, the researchers found that an immature form of red blood cell is instrumental in toning down immune reactions against microbes. These still-developing cells, which have a protein on their surface called CD71, also show up in abundance in the umbilical cord blood of human babies, Way’s team found. In mice, they appear to orchestrate immune suppression by making an enzyme called arginase-2, which in turn sends “stop” signals to inflammation triggers in the immune system.
In a series of experiments, the scientists found many more CD71 cells in 6-day-old neonatal mice than in adult mice, resulting in lower levels of inflammatory proteins in the neonates. That left the young mice hospitable to newly arriving microbes. When the scientists depleted the animals’ CD71 cells, a vigorous immune defense ensued against microbes in general, indicating that high levels of CD71 cells play a role in allowing commensal bacteria to colonize internal organs.
This state of immune tolerance toward microbes wore off in the mice over three weeks — a period that corresponds to one to two months in humans, Way estimates. At 9 days the animals still had high levels of CD71 cells, but by 15 days of age they had less than half as many as they had only a week earlier. At 21 days, their CD71 cell numbers equaled those of 8-week-old adult mice, contributing little to immune suppression.
“This is great. It’s a beautiful piece of work,” says Mike McCune, an infectious disease physician at the University of California, San Francisco. Doctors might someday be able to adjust immune suppression in babies who have too much or too little, enabling a newborn to welcome more commensal bacteria or fend off pathogens that pose risks.
Newborn immunity is controlled by more than just CD71 cells, in part because breast milk from the mother supplies some immune protection. But these scientists agree that the new immune suppression findings suggest an evolutionary trade-off with big returns. Infections are common in newborns, Way says, “and maybe 1 or 2 percent of newborns get seriously infected. But 100 percent need to be colonized with commensal bacteria.”