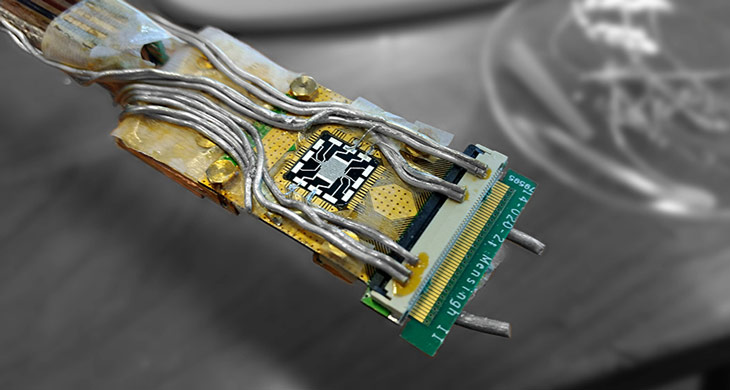
Scientists have chilled tiny electronics to a record low temperature
For the first time, nanoelectronics have been cooled to below a thousandth of a kelvin

GO COLD A miniature electronic chip, shown close up in a scanning electron microscope image, has been cooled to a temperature less than a thousandth of a kelvin.
QuTech/TUDelft