- More than 2 years ago
Editor’s note: On February 5, 2016, the journal Science retracted the research paper reported below. (See abstract of original paper.)
Cells employ RNA to make proteins, but now materials scientists have figured out how to use these genetic molecules for making metallic nanoparticles. The feat could open new avenues for producing inorganic materials, tailored on the nanoscale, for constructing such devices as fuel cells and quantum computers.
To create the particles, chemists Bruce Eaton and Dan Feldheim of North Carolina State University in Raleigh synthesized trillions of RNA fragments, each with its own sequence of building blocks called nucleotides. The fragments were chemically modified to bind to palladium. When added to a solution containing palladium atoms, some of the RNA fragments spontaneously organized those atoms into particles.
The researchers then separated the particle-building RNA fragments from those that didn’t produce anything. The sequences that produced the largest particles in the shortest time were transferred to another solution of palladium for a second round of selection.
In an engineering strategy known as directed evolution, the team repeated the cycle eight times. RNA fragments derived from the winners of the last cycle formed particles in a minute or less. That’s “pretty phenomenal,” Eaton adds.
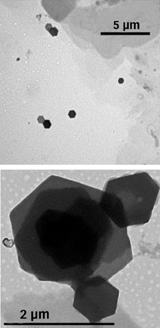
When the researchers examined the particles with an electron microscope, they were amazed. Instead of having varying shapes, almost all the particles were hexagonal platelets 1 to 2 micrometers in diameter and 20 nanometers thick, Feldheim and Eaton report in an upcoming Science.
Just how the process generates hexagonal particles remains unclear, but Feldheim suspects it has to do with the way RNA molecules form three-dimensional shapes.
“RNAs that fold just right can take those first few palladium atoms and put them together really quickly,” he says. This yields seed clusters that determine each particle’s final crystal structure.
Chad Mirkin of Northwestern University in Evanston, Ill., calls the approach “very clever.” The grand challenge of nanotechnology, he says, is to manipulate materials on the nanoscale. Says Mirkin: “If you can control a particle’s composition, size, and shape, you can control its chemical and physical properties.”
Other groups have used proteins to grow metallic nanoparticles and other inorganic structures, such as semiconducting nanowires (SN: 7/5/03, p. 7: Microbial Materials).
However, RNA could offer a faster and simpler route to making these materials, the North Carolina team suggests.
“RNA is very good at positioning metals,” notes Gerald Joyce of the Scripps Research Institute in La Jolla, Calif. With RNA, he adds, researchers can generate large numbers of nucleotide sequences and select just those that do the jobs they want.
By selecting for different traits, directed evolution could yield a diversity of inorganic shapes, among them cubes and wires, says Eaton. Since shape is central to a catalyst’s function, it might be possible, for example, to create palladium nanoparticles that split hydrogen from water for powering fuel cells. Or, with the help of a magnet, researchers might evolve RNA that produces particles of semiconducting magnetic materials for use in quantum computers.