Lost memories retrieved for mice with signs of Alzheimer’s
Method shows where the ability to remember breaks down in early stages of the disease
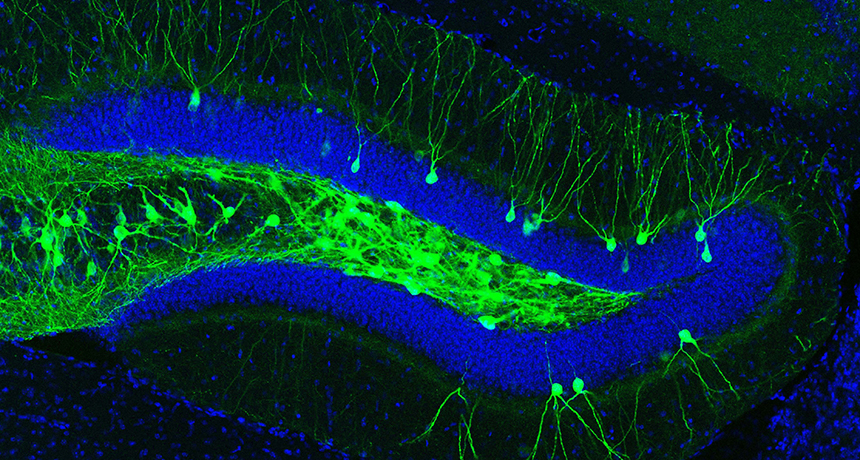
MEMORY SEEKER Nerve cells that hold a memory (green) in a mouse’s hippocampus can be coaxed into action with lasers, bringing the memory back up.
Dheeraj Roy
- More than 2 years ago
Using flashes of blue light, scientists have pulled forgotten memories out of the foggy brains of mice engineered to have signs of early Alzheimer’s disease. This memory rehab feat, described online March 16 in Nature, offers new clues about how the brain handles memories, and how that process can go awry.
The result “provides a theoretical mechanism for reviving old, forgotten memories,” says Yale School of Medicine neurologist Arash Salardini. Memory manipulations, such as the retrieval of lost memories and the creation of false memories, were “once the realm of science fiction,” he says. But this experiment and other recent work have now accomplished these feats, at least in rodents (SN: 12/27/14, p. 19), he says.
To recover a lost memory, scientists first had to mark it. Neuroscientist Susumu Tonegawa of MIT and colleagues devised a system that tagged the specific nerve cells that stored a memory — in this case, an association between a particular cage and a shock. A virus delivered a gene for a protein that allowed researchers to control this collection of memory-holding nerve cells. The genetic tweak caused these cells to fire off signals in response to blue laser light, letting Tonegawa and colleagues call up the memory with light delivered by an optic fiber implanted in the brain.
A day after receiving a shock in a particular cage, mice carrying two genes associated with Alzheimer’s seemed to have forgotten their ordeal; when put back in that cage, these mice didn’t seem as frightened as mice without the Alzheimer’s-related genes. But when the researchers used light to restore this frightening memory, it caused the mice to freeze in place in a different cage. (Freezing in a new venue showed that laser activation of the memory cells, and not environmental cues, caused the fear reaction.)
The fact that this memory could be pulled out with light helps clarify the source of memory trouble for people with Alzheimer’s, Tonegawa says. In this experiment, the mice appeared able to form and store a memory but not call it up. “It’s a retrieval problem, not a storage problem,” Tonegawa says.
That’s in line with what many clinicians now believe to be happening in early Alzheimer’s, says Salardini. People in the early stages of the disease seem able to create new memories, but then rapidly forget them, he says. Memories can sometimes be strengthened with reminders and clues from the environment, suggesting that they are “somewhere in there,” but not retrievable, he says.
Further experiments with the mice showed that the fear memory could be strengthened by forcing it to appear multiple times. This memory boot camp worked because it boosted the number of docking sites on memory-holding nerve cells in the mice with Alzheimer’s-related genes. Usually, these docking sites — knobs called dendritic spines that receive messages from other nerve cells — become scarcer with age. To counter that, Tonegawa and colleagues used light to repeatedly activate nerve cells that in turn activate the memory-holding cells. Compared with mice that didn’t get this strengthening treatment, mice with the Alzheimer’s genes that underwent this process were more fearful of the cage where they had received a shock, even six days later.
Tonegawa cautions that the results are experimental. “We have not done anything to cure human Alzheimer’s patients,” he says. And the methods, which rely on viruses to genetically engineer brain cells and optic fibers implanted in the brain, are not currently feasible for people.
But insights gained from this experiment, and others like it, do help clarify how memory works in people, says neuroscientist Christine Denny of Columbia University. “If we can understand how the process of memory retrieval is compromised and where it is impaired, then we can begin to develop treatments to target those processes or circuits.”