A key virus fighter is implicated in pregnancy woes
Fetal mice whose immune system revved up in response to their mom’s Zika infection died or grew poorly
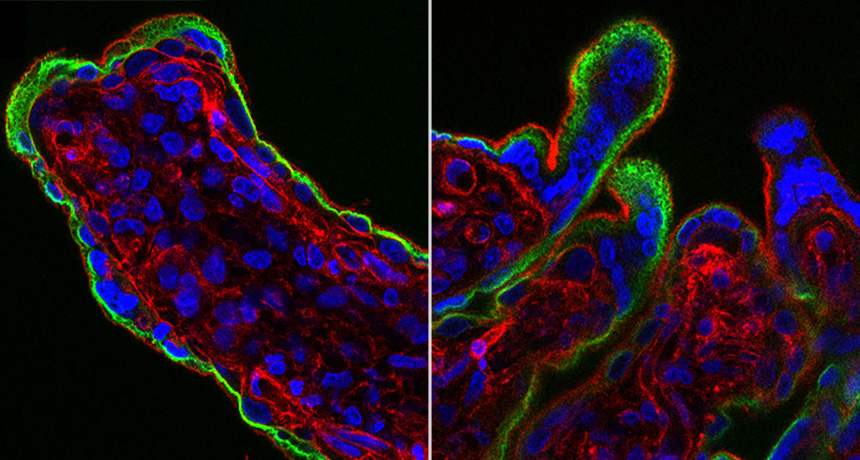
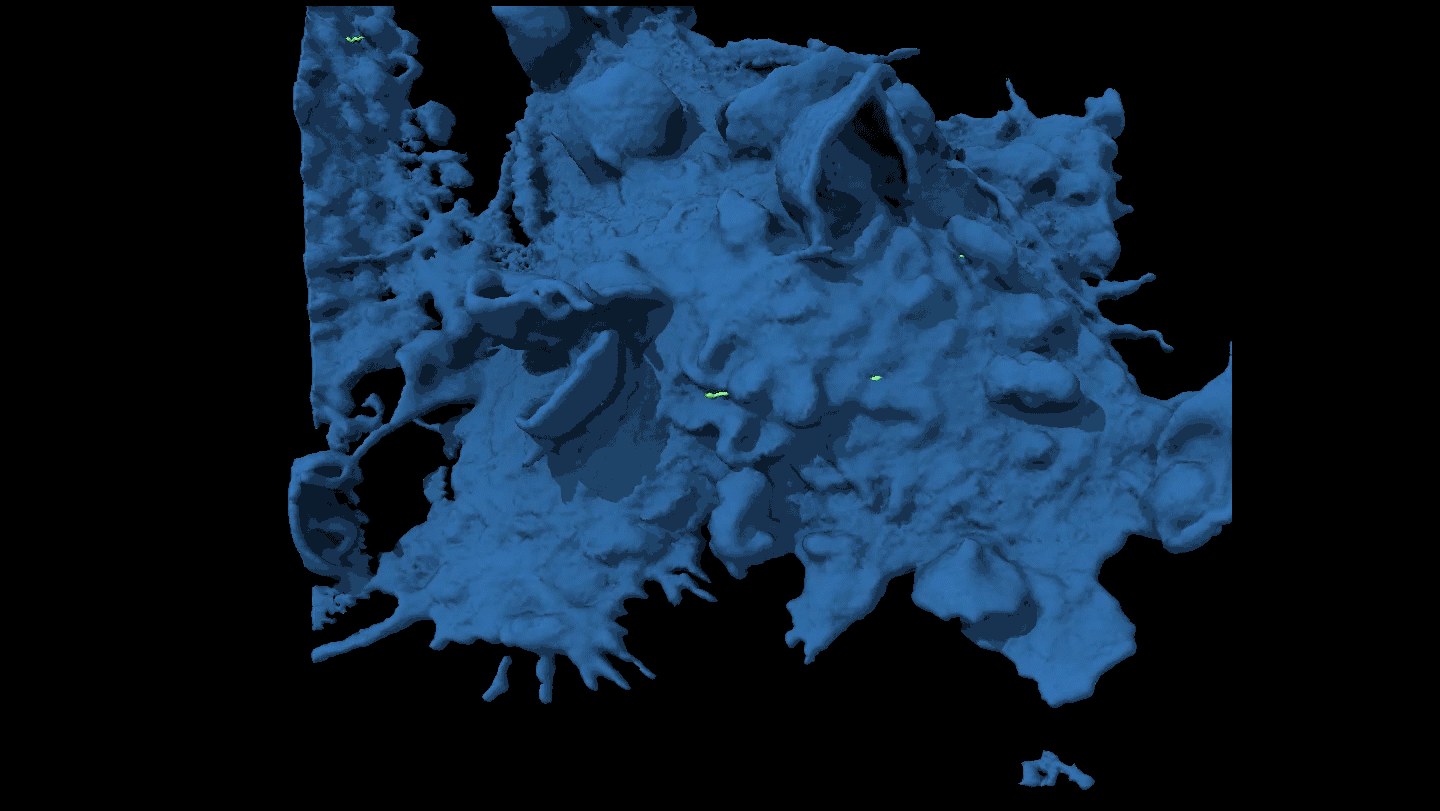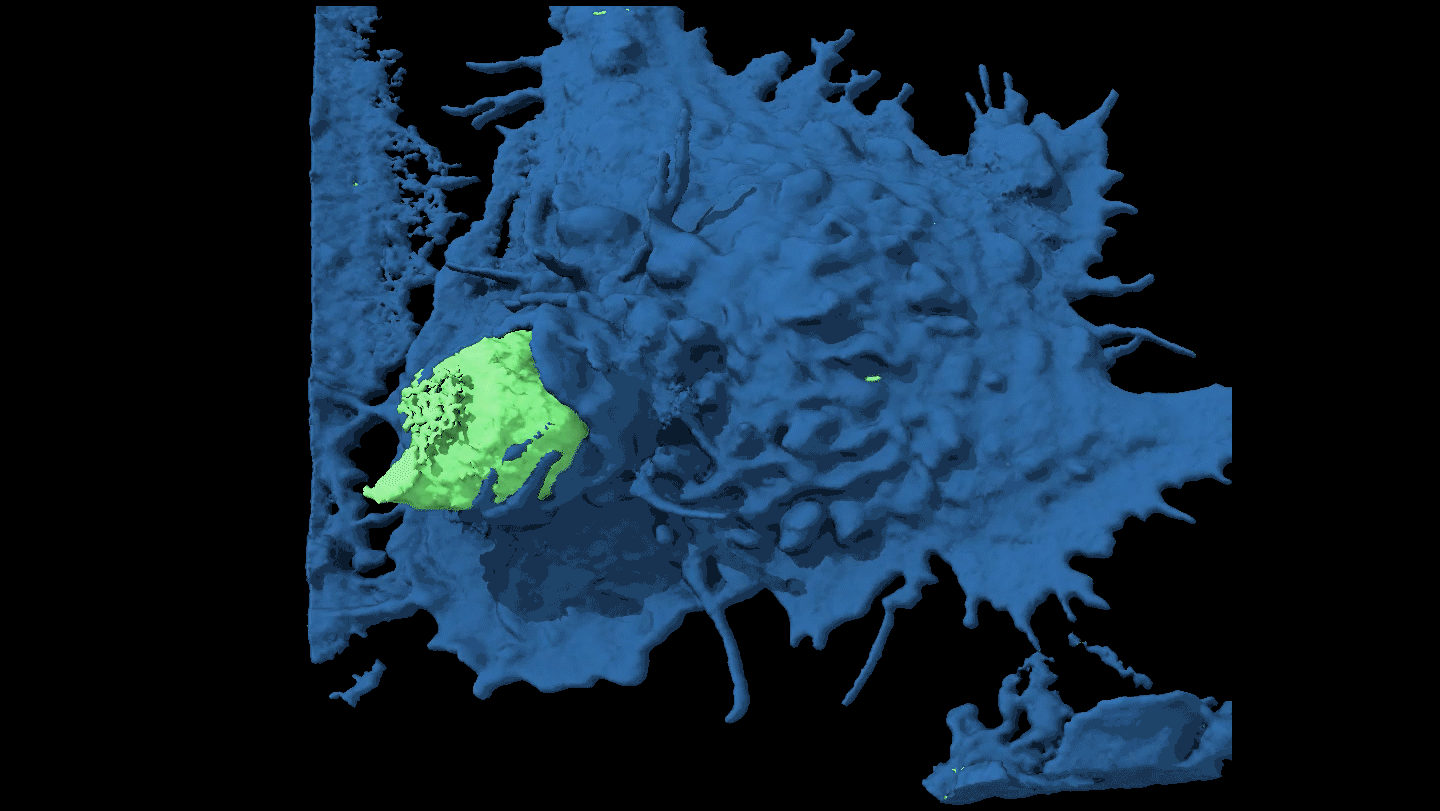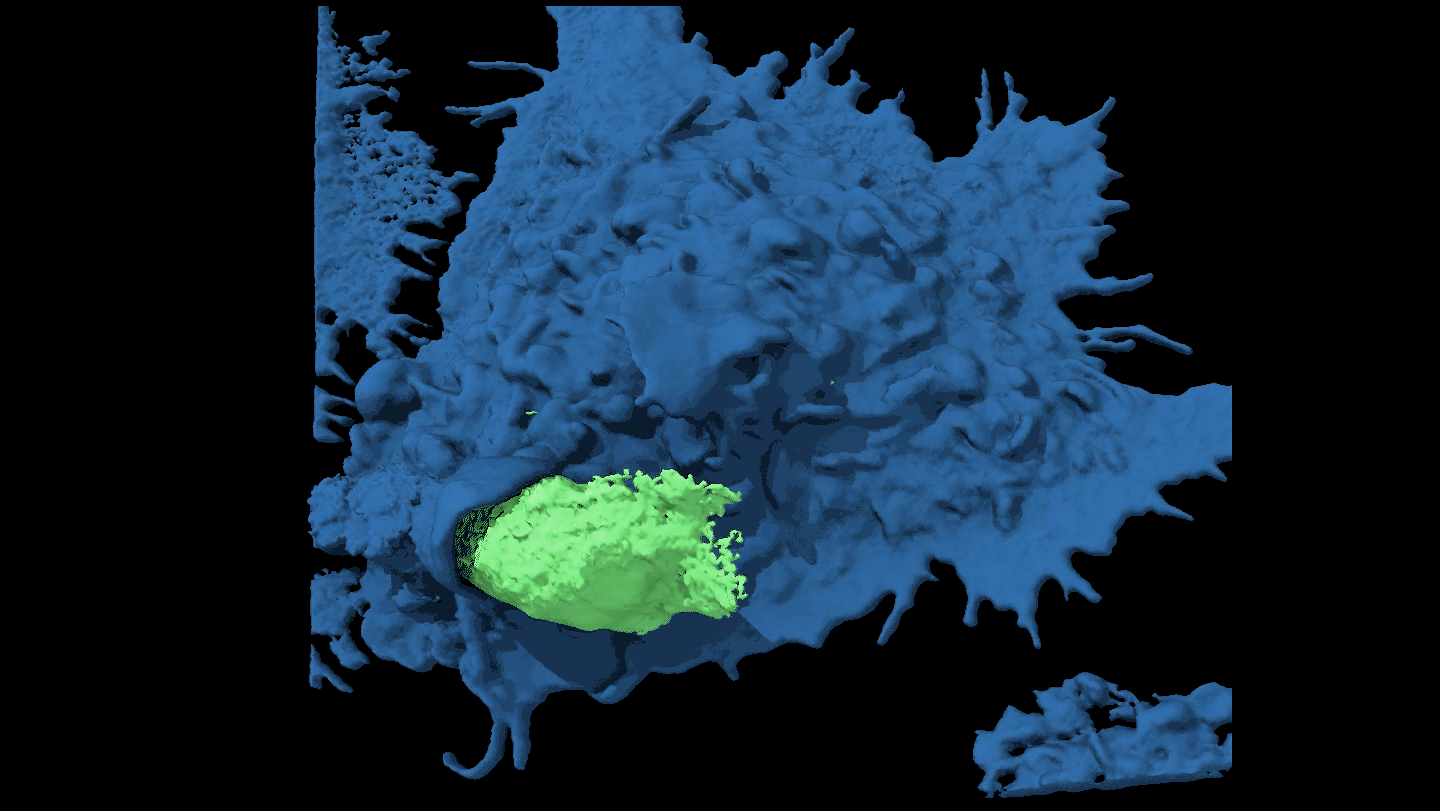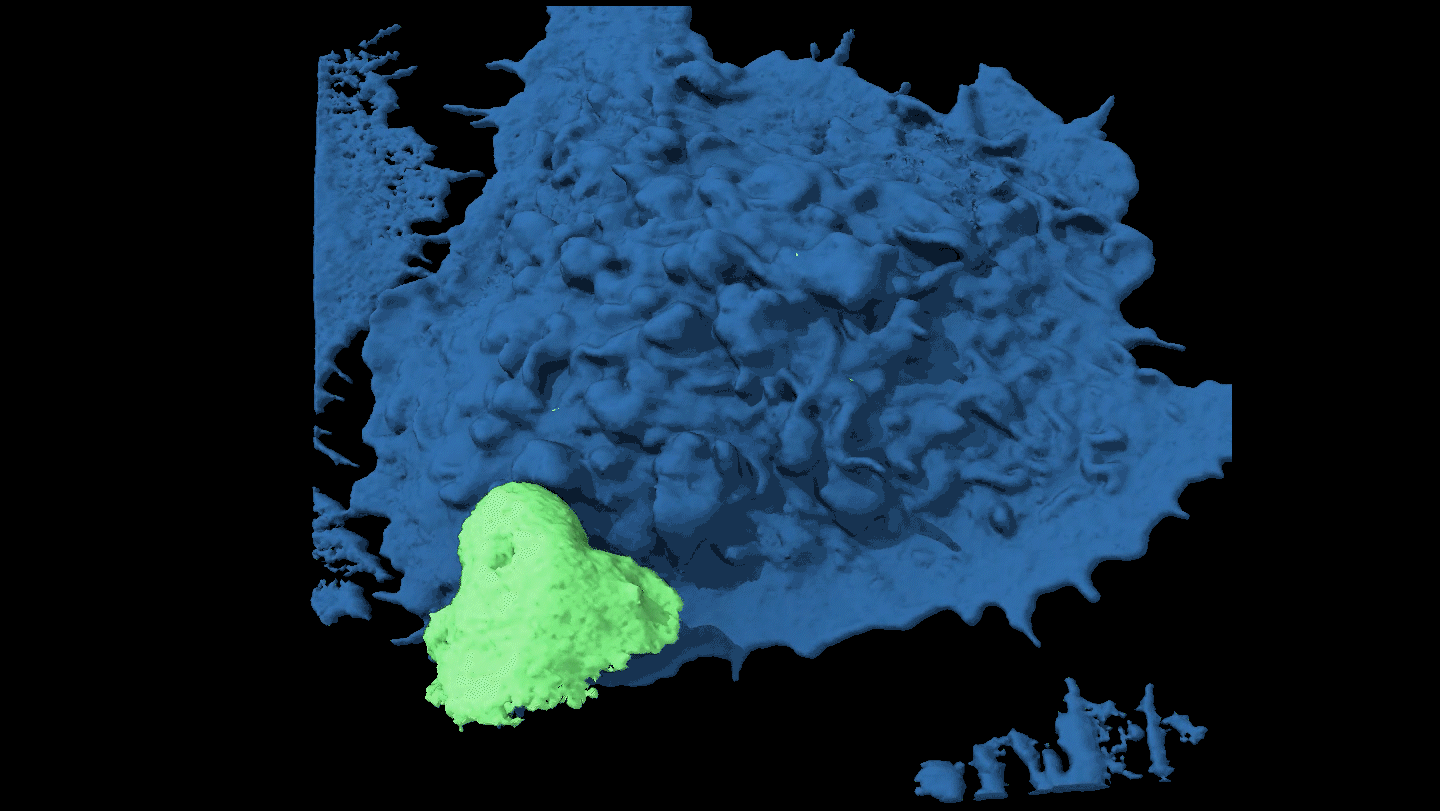
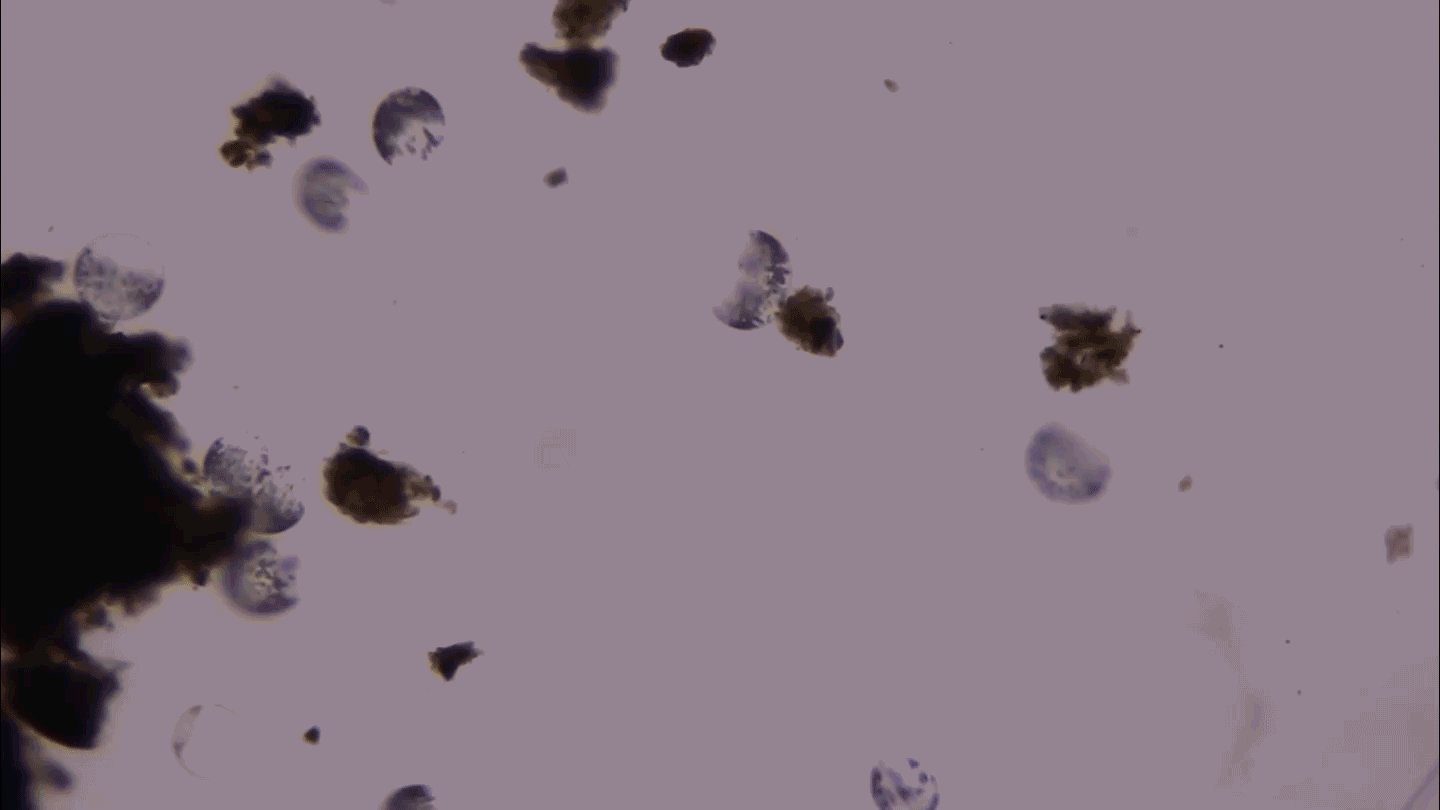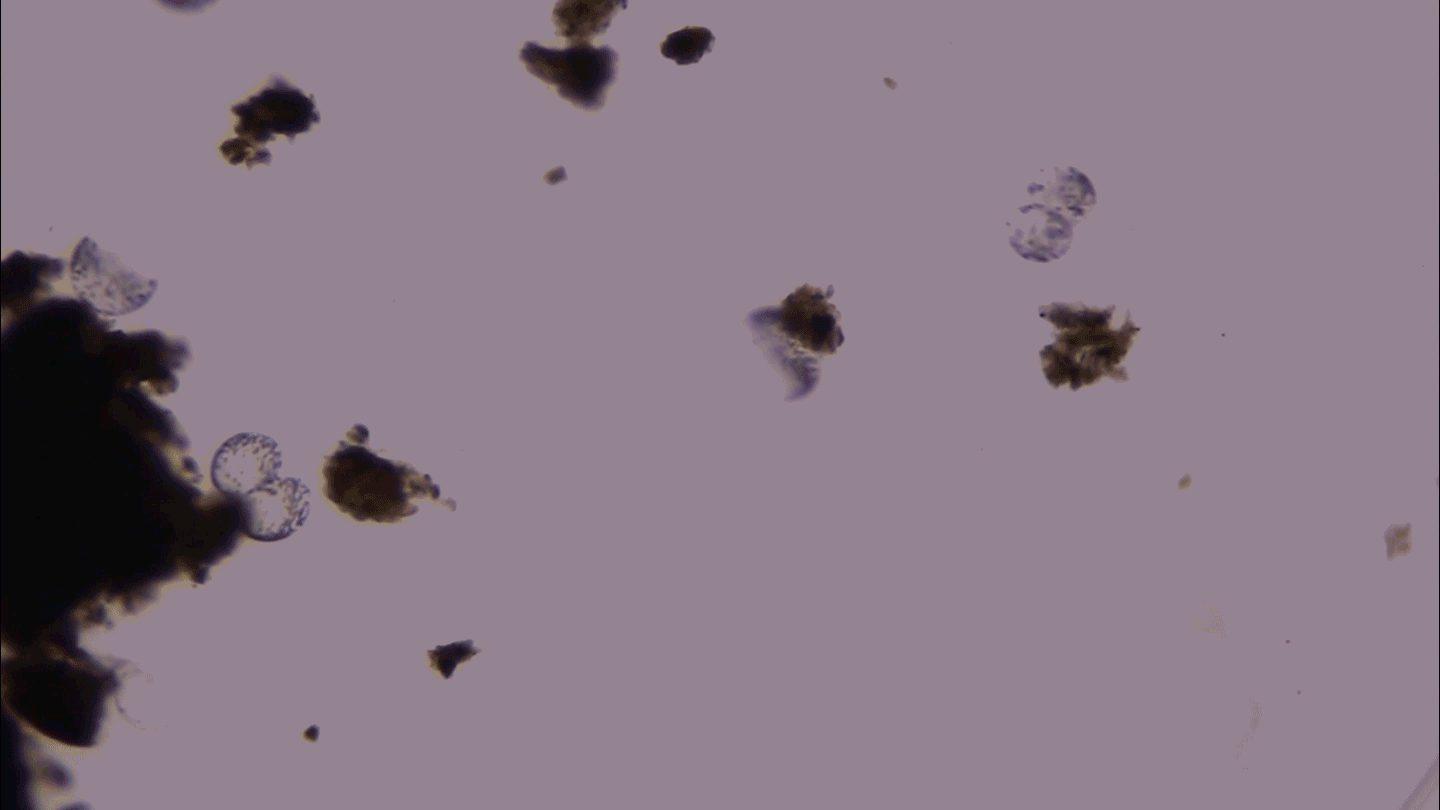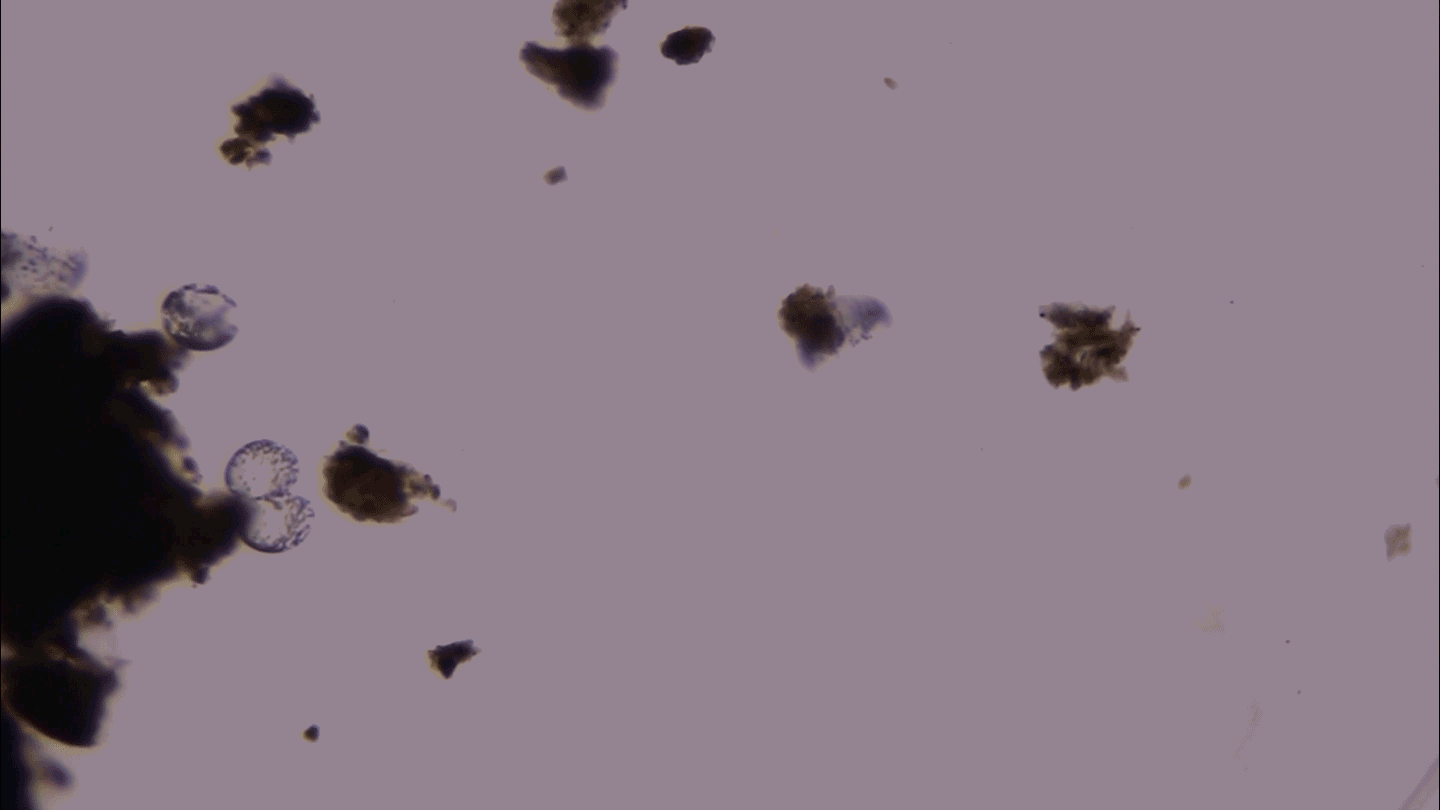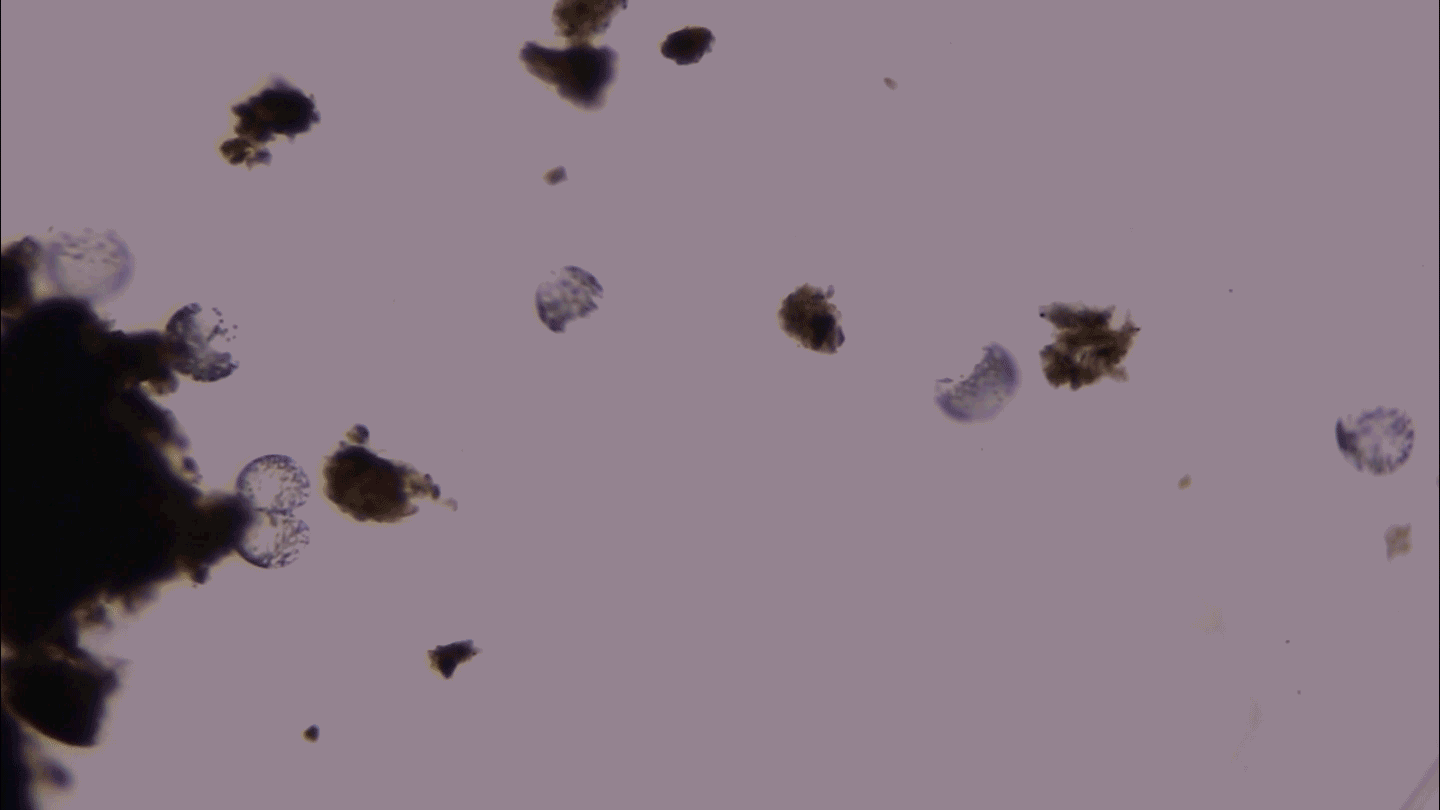
KNOTTY ISSUES Placental tissue (on the right) treated with an immune protein that fights viruses developed knotty structures that resembled those associated with pregnancy complications. Untreated placental tissue (on the left) does not show these structures.
L. Yockey et al/Science Immunology 2018, Carolyn Coyne
- More than 2 years ago
An immune system mainstay in the fight against viruses may harm rather than help a pregnancy. In Zika-infected mice, this betrayal appears to contribute to fetal abnormalities linked to the virus, researchers report online January 5 in Science Immunology. And it could explain pregnancy complications that arise from infections with other pathogens and from autoimmune disorders.
In pregnant mice infected with Zika virus, those fetuses with a docking station, or receptor, for immune system proteins called type I interferons either died or grew more poorly compared with fetuses lacking the receptor. “The type I interferon system is one of the key mechanisms for stopping viral infections,” says Helen Lazear, a virologist at the University of North Carolina at Chapel Hill, who coauthored an editorial accompanying the study. “That same [immune] process is actually causing fetal damage, and that’s unexpected.”
Cells infected by viruses begin the fight against the intruder by producing type I interferons. These proteins latch onto their receptor on the surfaces of neighboring cells and kick-start the production of hundreds of other antiviral proteins.
Akiko Iwasaki, a Howard Hughes Medical Institute investigator and immunologist at Yale School of Medicine, and her colleagues were interested in studying what happens to fetuses when moms are sexually infected with Zika virus. The researchers mated female mice unable to make the receptor for type I interferons to males with one copy of the gene needed to make the receptor. This meant that moms would carry some pups with the receptor and some without in the same pregnancy.
Pregnant mice were infected vaginally with Zika at one of two times — one corresponding to mid‒first trimester in humans, the other to late first trimester. Of the fetuses exposed to infection earlier, those that had the interferon receptor died, while those without the receptor continued to develop. For fetuses exposed to infection a bit later in the pregnancy, those with the receptor were much smaller than their receptor-lacking counterparts.
Story continues below graphic
With or without
Pregnant mice were infected with Zika virus early in pregnancy (corresponding to mid–first trimester in humans). After 12 and a half days, which roughly corresponds to the second trimester in humans, all of the mouse fetuses with the interferon receptor had died, while those without it continued to develop.
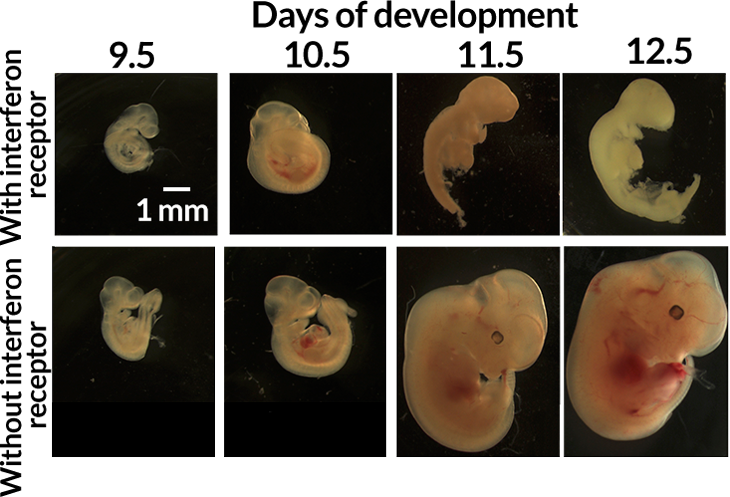
The fetuses without the receptor still grew poorly due to the Zika infection, which is expected given their inability to fight the infection. What was striking, Iwasaki says, is that the fetuses able to fight the infection were more damaged, and were the only fetuses that died.
It’s unclear how this antiviral immune response causes fetal damage. But the placentas—which, like their fetuses, had the receptor — didn’t appear to provide those fetuses with enough oxygen, Iwasaki says.
The researchers also infected pregnant mice that had the receptor for type I interferons with a viral mimic — a bit of genetic material that goads the body to begin its antiviral immune response — to see if the damage happened only during a Zika infection. These fetuses also died early in the pregnancy, an indication that perhaps the immune system could cause fetal damage during other viral infections, Iwasaki notes.
Iwasaki and colleagues next added type I interferon to samples of human placental tissue in dishes. After 16 to 20 hours, the placental tissues developed structures that resembled syncytial knots. These knots are widespread in the placentas of pregnancies with such complications as preeclampsia and restricted fetal growth.
Figuring out which of the hundreds of antiviral proteins made when type I interferon ignites the immune system can trigger placental and fetal damage is the next step, says Iwasaki. That could provide more understanding of miscarriage generally; other infections that cause congenital diseases, like toxoplasmosis and rubella; and autoimmune disorders that feature excessive type I interferon production, such as lupus, she says.