SPACE SUGAR Ribose, the sugar in RNA, might form on ice grains irradiated by ultraviolet light in stellar nurseries such as the Carina Nebula, one part of which is seen in this Hubble Space Telescope image.
NASA, ESA, M. Livio, Hubble 20th Anniversary Team/STScI
- More than 2 years ago
Joni Mitchell was right: We are stardust. Another one of the essential ingredients for life as we know it might have formed in space and then rained down on a young Earth, researchers suggest in the April 8 Science.
The simple sugar ribose — a crucial piece of the molecular machinery inside cells — can form on a blend of ices that have been blasted with ultraviolet radiation, chemist Cornelia Meinert of the University Nice Sophia Antipolis in France and colleagues report. These ices are common to comets and are thought to coat grains of interstellar dust that swirl around young stars.
“This is an amazing result,” says Conel Alexander, a planetary scientist at the Carnegie Institution for Science in Washington, D.C. Researchers have been looking at irradiated ices for years, he says, but have never seen ribose show up. Ribose is the backbone of RNA, a molecule that helps carry out the instructions encoded in genes. RNA is found in all life on Earth and might have been a precursor to DNA billions of years ago.
Other pieces of biological machinery have been found in space or produced in a lab before. Last year, researchers created uracil, cytosine and thymine — three of the molecular letters in DNA’s and RNA’s genetic alphabets — in a simulated space environment. Researchers have also found amino acids, which link together to form proteins, in meteorites. And in 2014, astronomers detected isopropyl cyanide, a molecule that resembles amino acids, in a gas cloud near the center of the galaxy (SN: 11/1/14, p. 7). A decade earlier, scientists reported finding the simple sugar glycoaldehyde in the same cloud (SN: 10/9/04, p. 237).
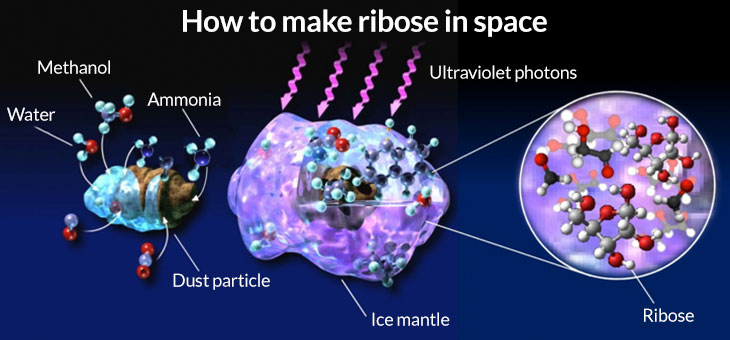
Meinert and colleagues cooled water, ammonia and methanol, which are ices found in comets, to –195° Celsius inside a vacuum chamber. To simulate the radiation from a young star, the researchers then exposed the ice to ultraviolet light. After warming the cosmic goop back to room temperature, they found that a cornucopia of organic molecules — about 55 in all, including ribose — had formed on the ice.
The results aren’t too surprising, says Reggie Hudson, an astrochemist at NASA’s Goddard Space Flight Center in Greenbelt, Md. Researchers have long suspected that sugars could form on interstellar ice, and the underlying chemistry has been understood for 155 years, he says, but no one had actually done the experiment before.
One of the challenges is that the compounds that formed are also common contaminants. People carry many of the sugars around in their bodies. And one of the molecules that turned up is ethylene glycol, also known as antifreeze. Any of these could have been inadvertently introduced to the experiment. But the researchers used methanol ice that contained a variety of carbon known as carbon-13; any contaminants would have carried the slightly lighter carbon-12. By seeing carbon-13 show up in the ribose and other sugars, the researchers knew chemical reactions in the ice and not uninvited interlopers were responsible for the results.
Hudson also notes — as do the study’s authors — that it’s hard to tell whether the molecules formed when the ices were cold or as the samples warmed up. But both Hudson and Meinert say that warm temperatures aren’t foreign to interstellar ice grains. Young stars will periodically warm up their surrounding belts of debris. And grains that fall to Earth will heat up as they pummel the planet. Ribose, instead of forming in space, might have formed as the ingredients were delivered to Earth.
No one has seen ribose in comets and asteroids yet, but a few upcoming missions might get a chance to look. Japan’s Hayabusa2 spacecraft is on course to pilfer material from asteroid 162173 Ryugu; NASA’s OSIRIS-REx mission meanwhile will launch in September to bring back pieces of asteroid Bennu.