Here’s how ice may get so slippery
Images of atoms on the surface of frozen water hint at how a slick layer forms
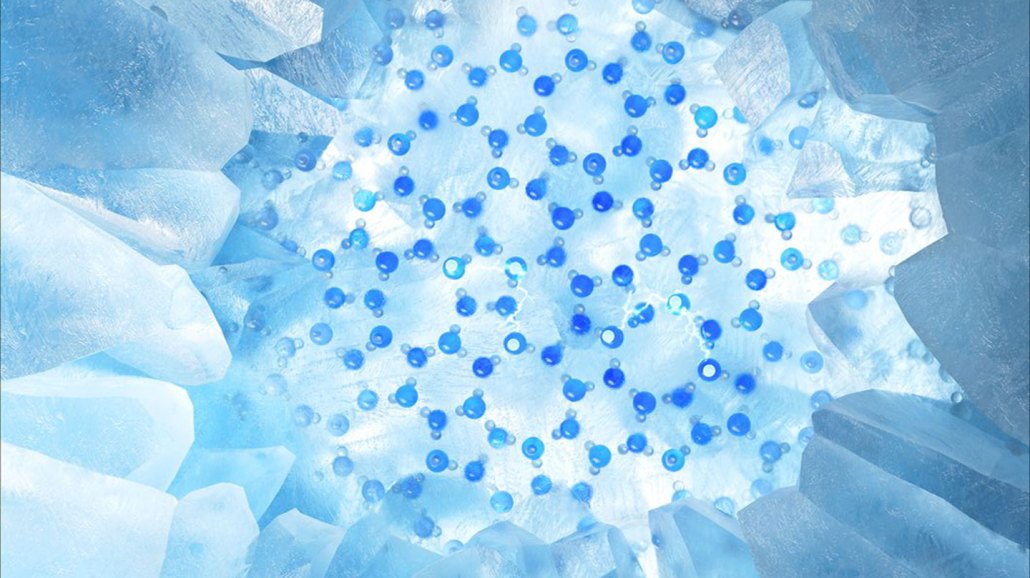
Frozen water’s slipperiness comes from a liquidlike layer on its surface. Arrangements of water molecules on the surface of ice (illustrated in blue) are helping to explain how that layer forms.
Jiang group/Peking University
The surface of ice is a slippery subject.
For more than 160 years, scientists have been debating the quirks of ice’s exterior. Frozen water is coated in a layer of molecules that behave like a liquid. A new experiment visualizes the surface of ice and hints at the origins of its quasi-liquid layer.
Ice’s melty coating appears even at temperatures well below freezing, a phenomenon known as “premelting.” That layer acts as a lubricant, explaining why ice is slippery even under frigid conditions. But ever since the idea of a liquidlike coating was first pondered by British scientist Michael Faraday in the 1850s, ice’s unusual surface has remained poorly understood.
In the new study, scientists used atomic force microscopy to measure the locations of atoms on the surface of ice. At temperatures around –150° Celsius, ice’s surface is made of not just one kind of ice, but two, physicist Ying Jiang of Peking University and colleagues report May 22 in Nature. What’s more, Jiang says, “ice is not so perfect.” The team found defects in the surface’s structure that seem to kick off the premelting.

Water molecules, consisting of one oxygen atom (blue, red or black) and two hydrogen atoms (white), can take on different structures on the surface of ice. Ice Ih (blue) consists of layers of water molecules arranged in hexagons stacked directly on top of one another. Ice Ic (red) is shifted relative to the layer below (indicated by hexagons). The surface of ice at low temperature is a mixture of both phases, with disordered molecules (black) in the borders between the two phases.
Jiang group/Peking UniversityIce comes in a variety of types, depending on the arrangement of its molecules (SN: 2/2/23). Under normal conditions, the water molecules are arranged in layers of hexagons stacked on top of one another. This hexagonal ice, called ice Ih, is the variety Jiang and colleagues studied. But the team found that the surface of the ice wasn’t entirely hexagonal. The atomic force microscope images revealed that the surface consisted of some regions of ice Ih and other regions of ice Ic, in which the hexagons in each layer are shifted to create a structure similar to the arrangement of carbon atoms in diamond.
“I was super impressed.… The picture is so beautiful,” says chemist Yuki Nagata of the Max Planck Institute for Polymer Research in Mainz, Germany. “It’s very hard to identify where the molecules are, but I think they very successfully get it.”

An atomic force microscope image reveals the structure of the surface of ice. Bright spots indicate the locations of water molecules. Brighter spots indicate water molecules with hydrogen atoms pointing up, and dimmer spots have hydrogen atoms lying flat or pointing down. Triangles pointing up surround ice in one phase, while triangles pointing down indicate another phase of ice.
J. Hong et al/Nature 2024At the borders between the two types of ice, the researchers found defects in the ice’s structure, resulting from the misalignment of the two patterns. When the researchers cranked up the temperature by a few degrees, those disordered regions expanded. In liquids, atoms and molecules are similarly jumbled, and the same goes for ice’s quasi-liquid layer. The expansion of the disorder marks the initial stages of premelting, the team argues.
As the temperature was raised further, structures in those borders amplified the disorder even more. Normally, ice is made up of crinkled layers: Some water molecules in each layer are lower, and some higher. But the team found places where water molecules lined up in a plane, a structure that served as a “seed” of yet more disorder.
At even higher temperatures, the team’s computer simulations suggest, the disorder would expand to cover the entire surface of the ice, giving ice its full liquidlike patina.
Figuring out the structure of ice’s surface required the ability to pinpoint locations of individual hydrogen atoms — each effectively just a single proton. That’s a task that’s normally challenging with atomic force microscopy. An atomic force microscope probes materials with a tip so thin that just one molecule or atom hangs at the end. The researchers added a special tweak, attaching a carbon monoxide molecule to the tip of the probe (SN: 4/28/20). By measuring the force between that tip and the surface as the tip scanned across the ice, the team could deduce the locations of the protons.
The study was performed at temperatures much lower than ice in everyday experience. But because the experiment must be done in a vacuum, raising the temperature too much would cause water molecules to escape from the ice. Eventually, the researchers hope to use short laser pulses to briefly heat the ice to get measurements under balmier conditions.