Gut fungi might be linked to obesity and inflammatory bowel disorders
Yeast and bacteria can team up to cause trouble
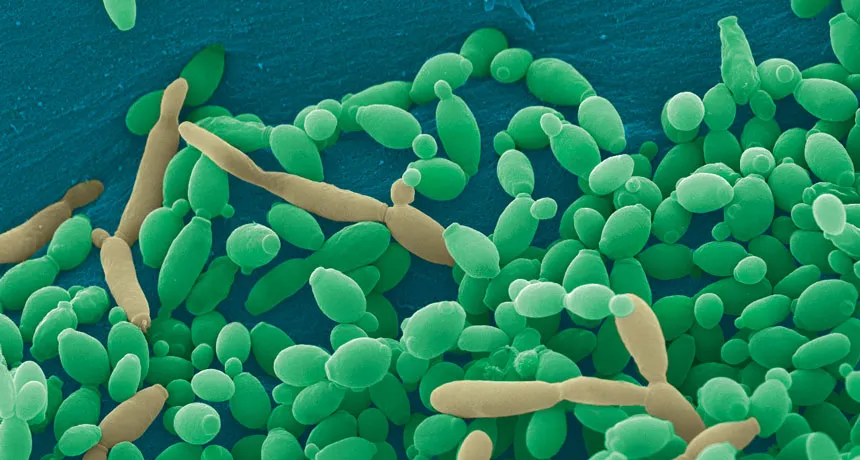
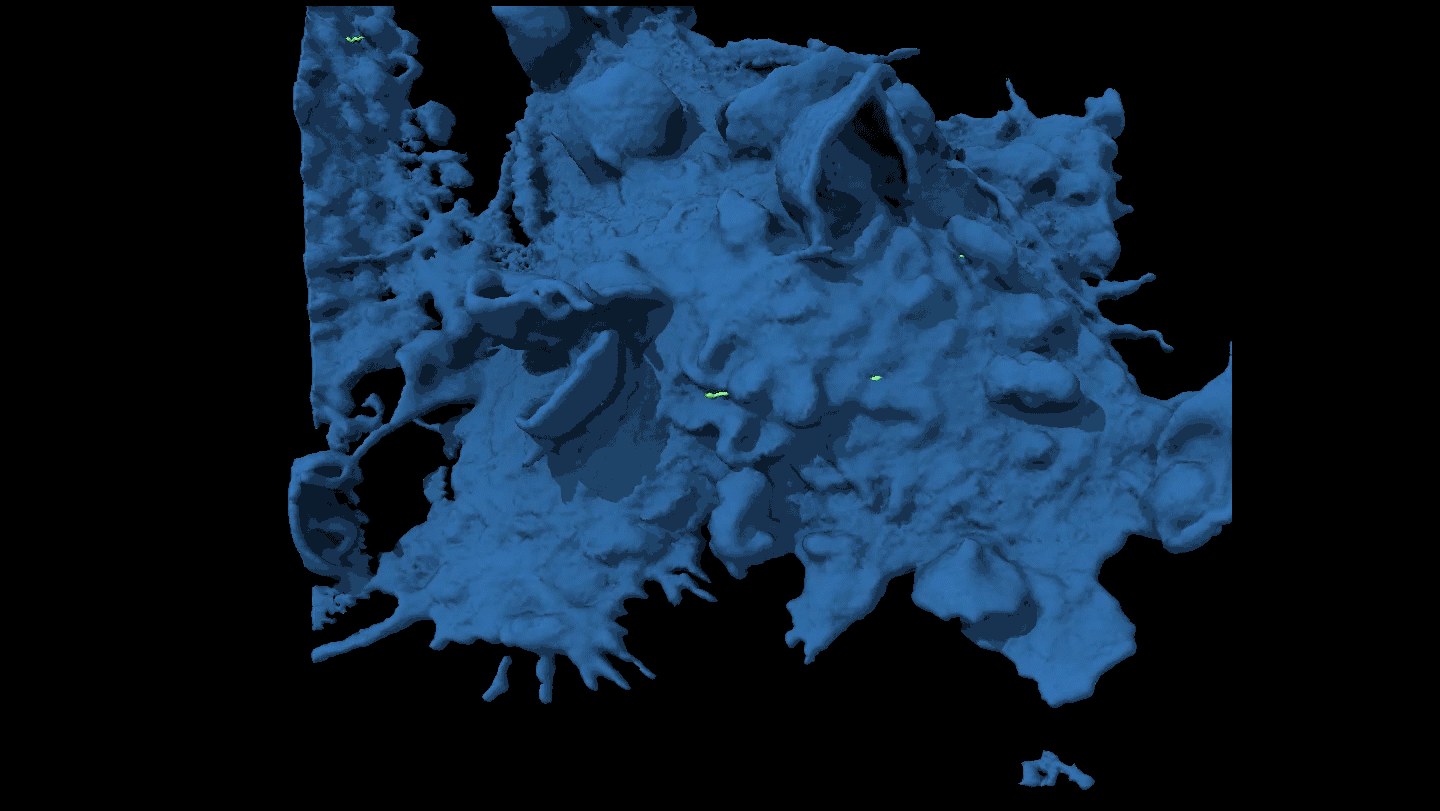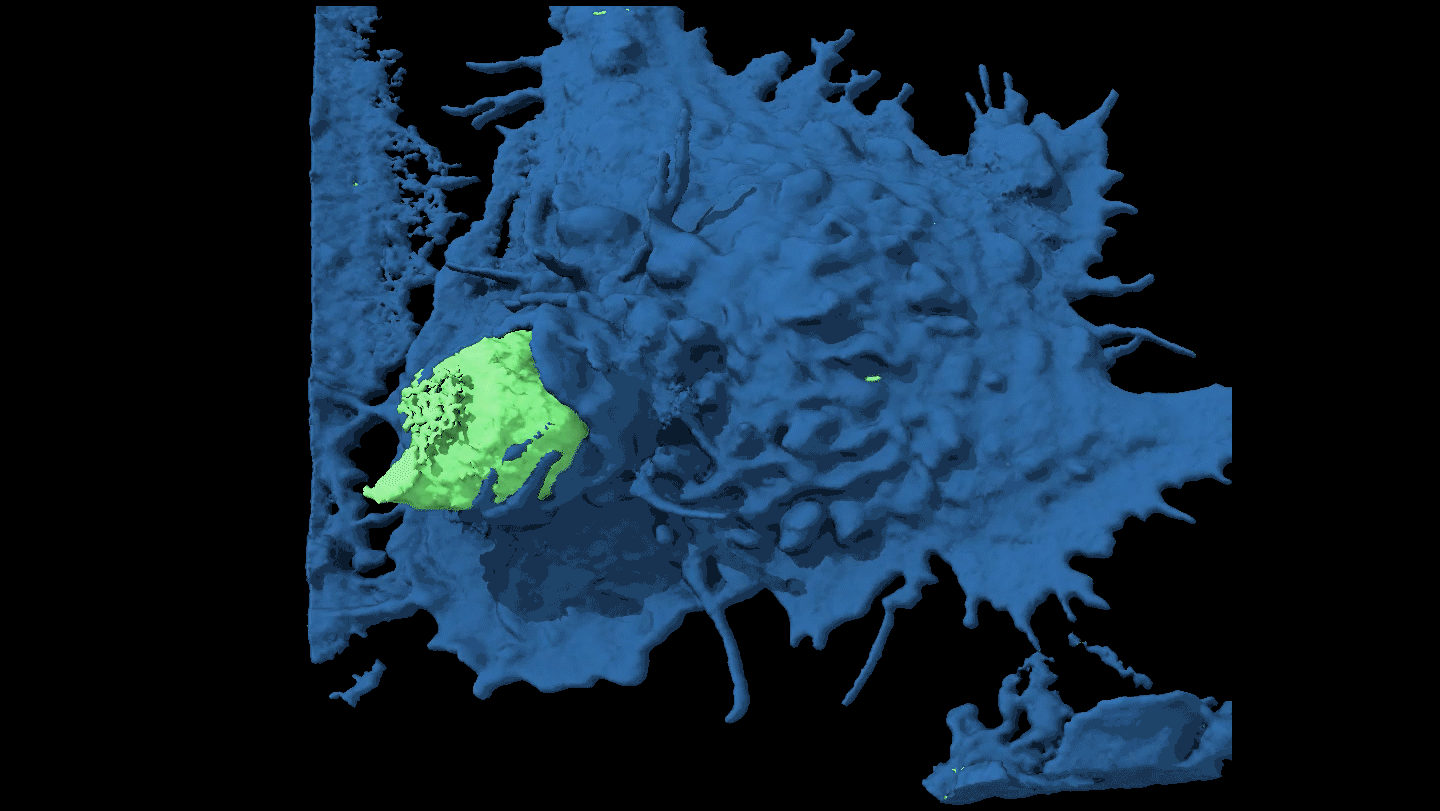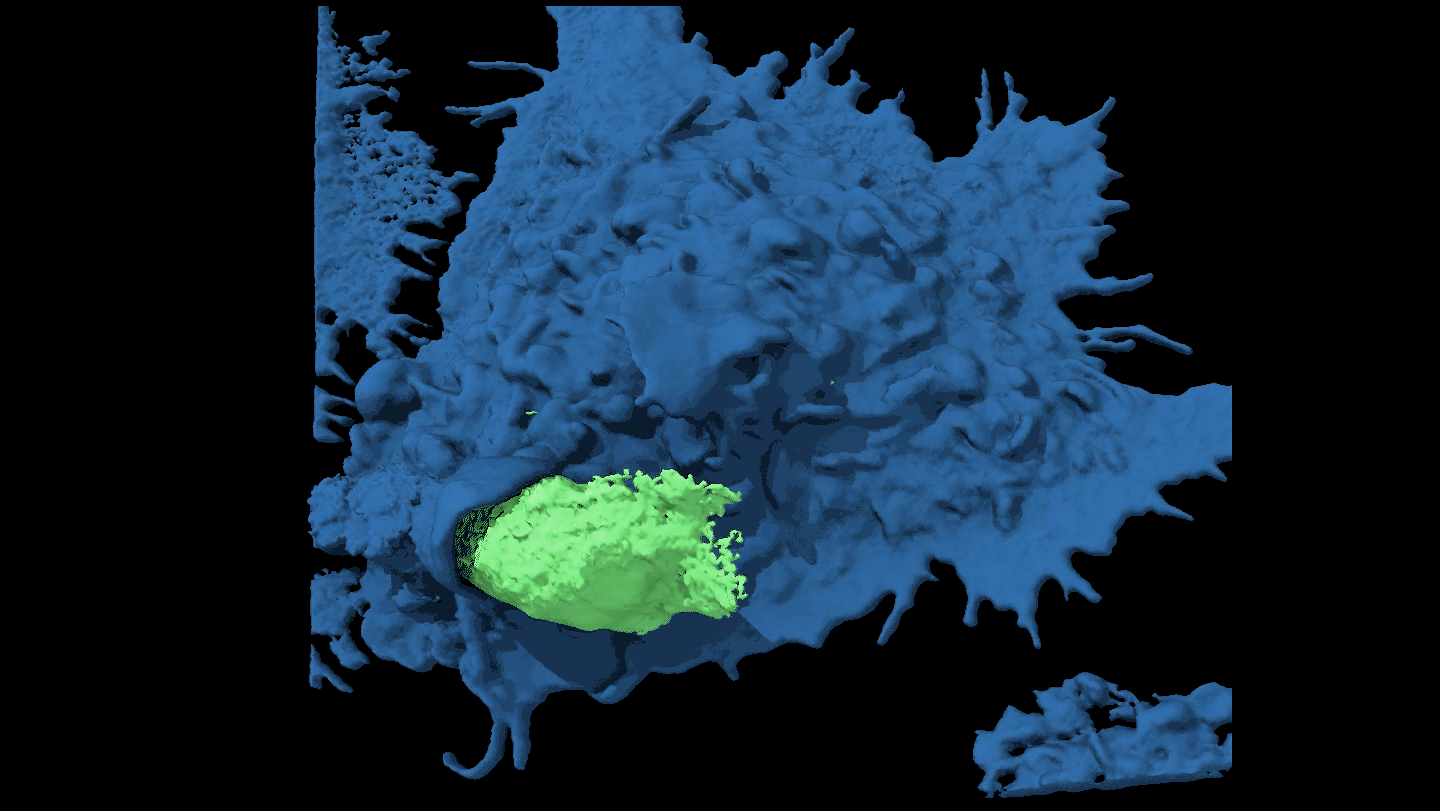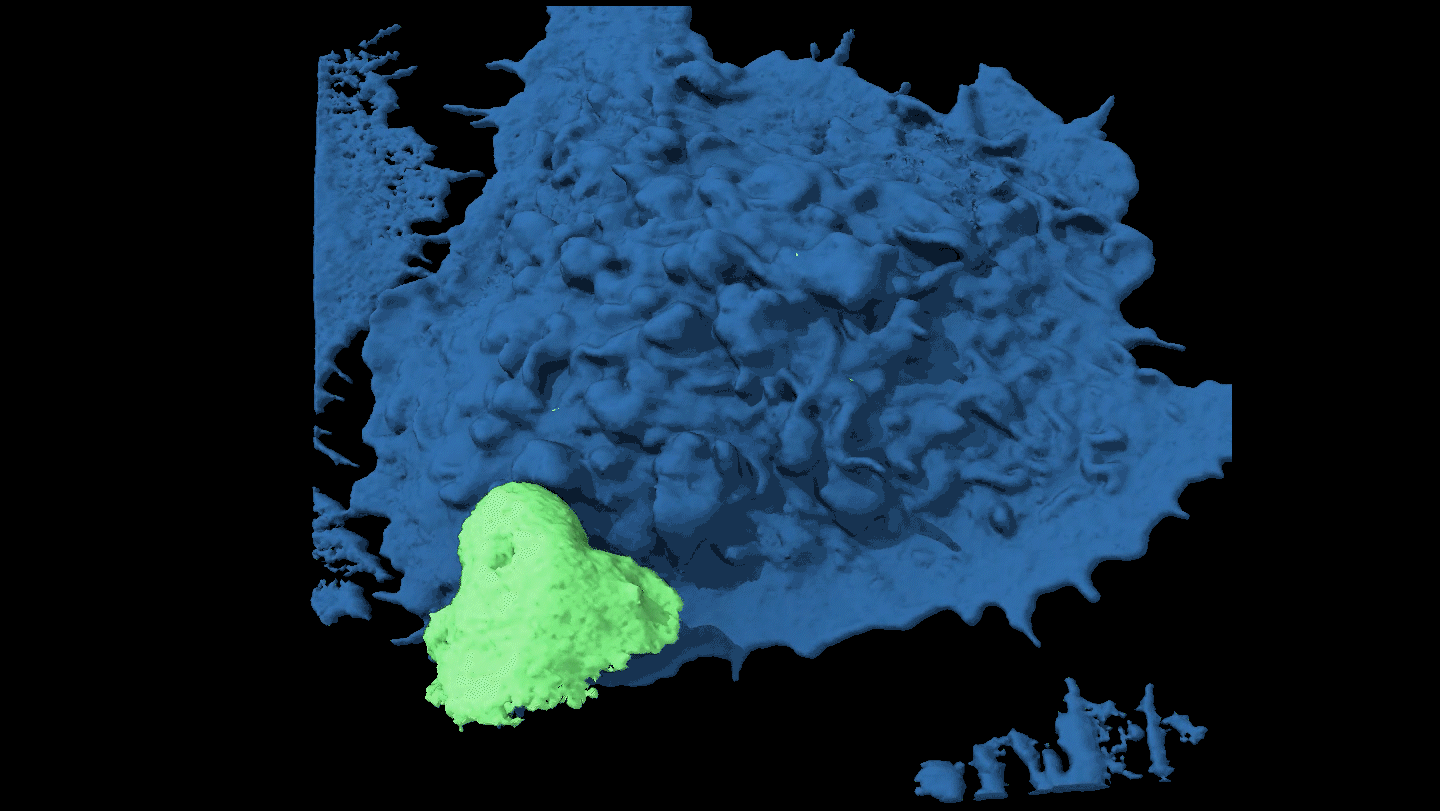
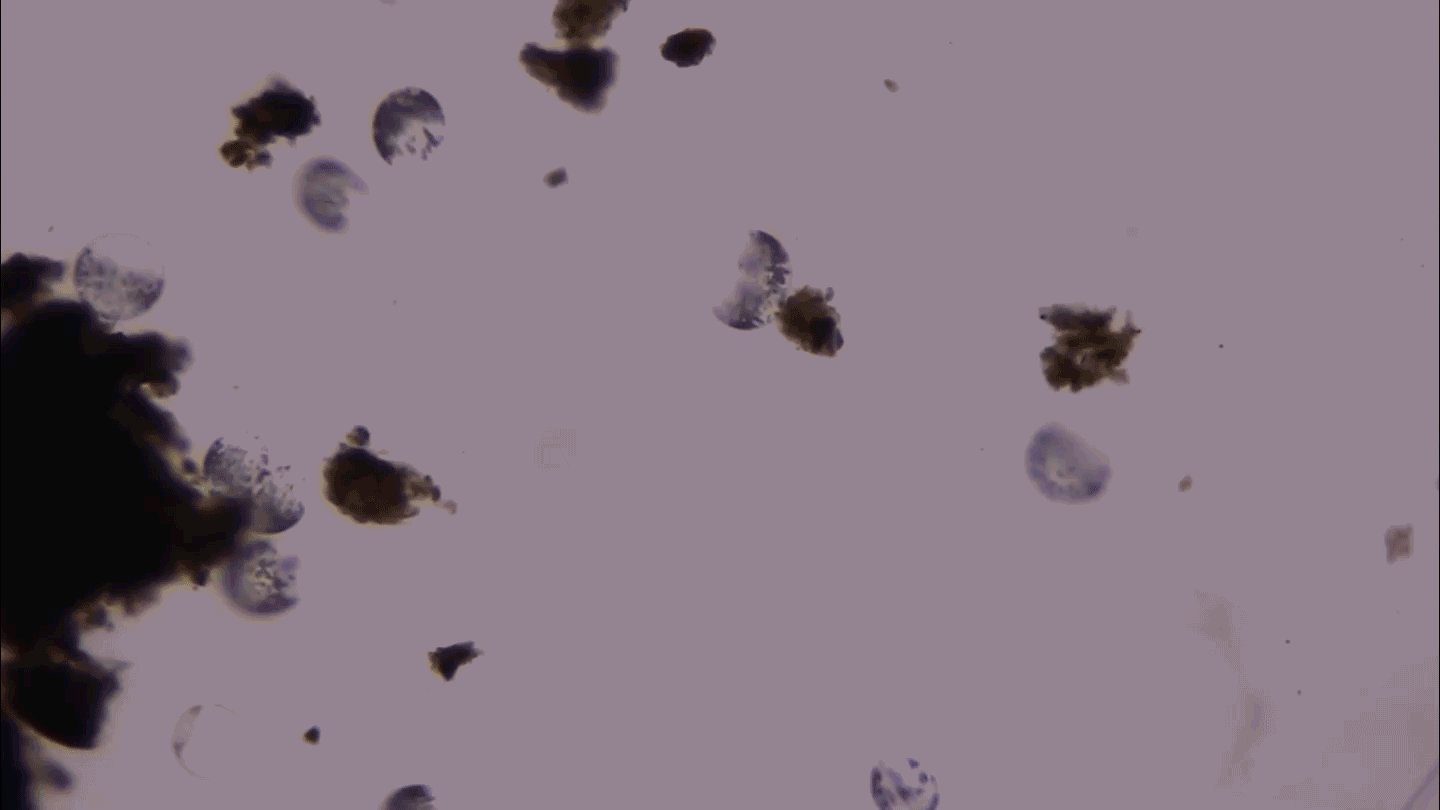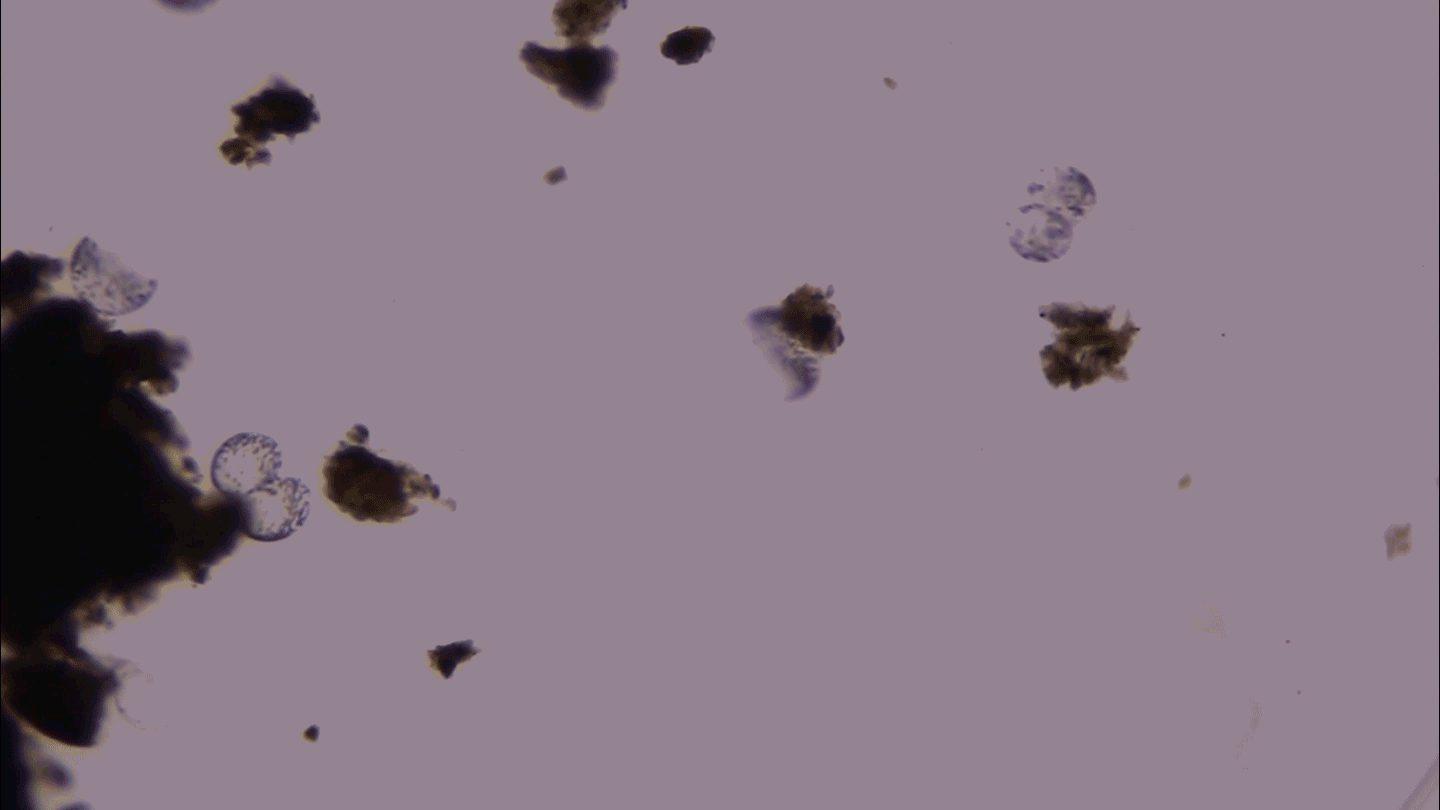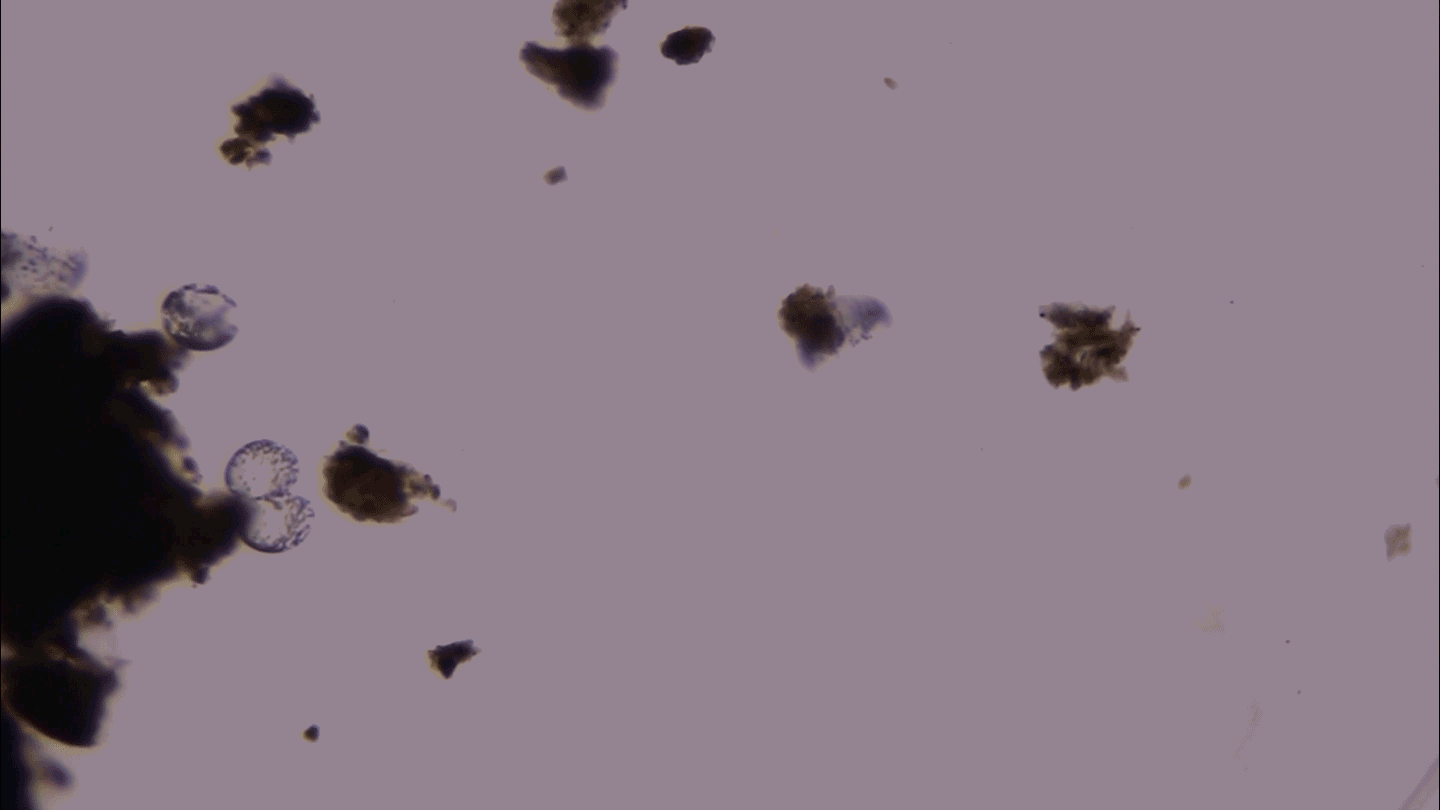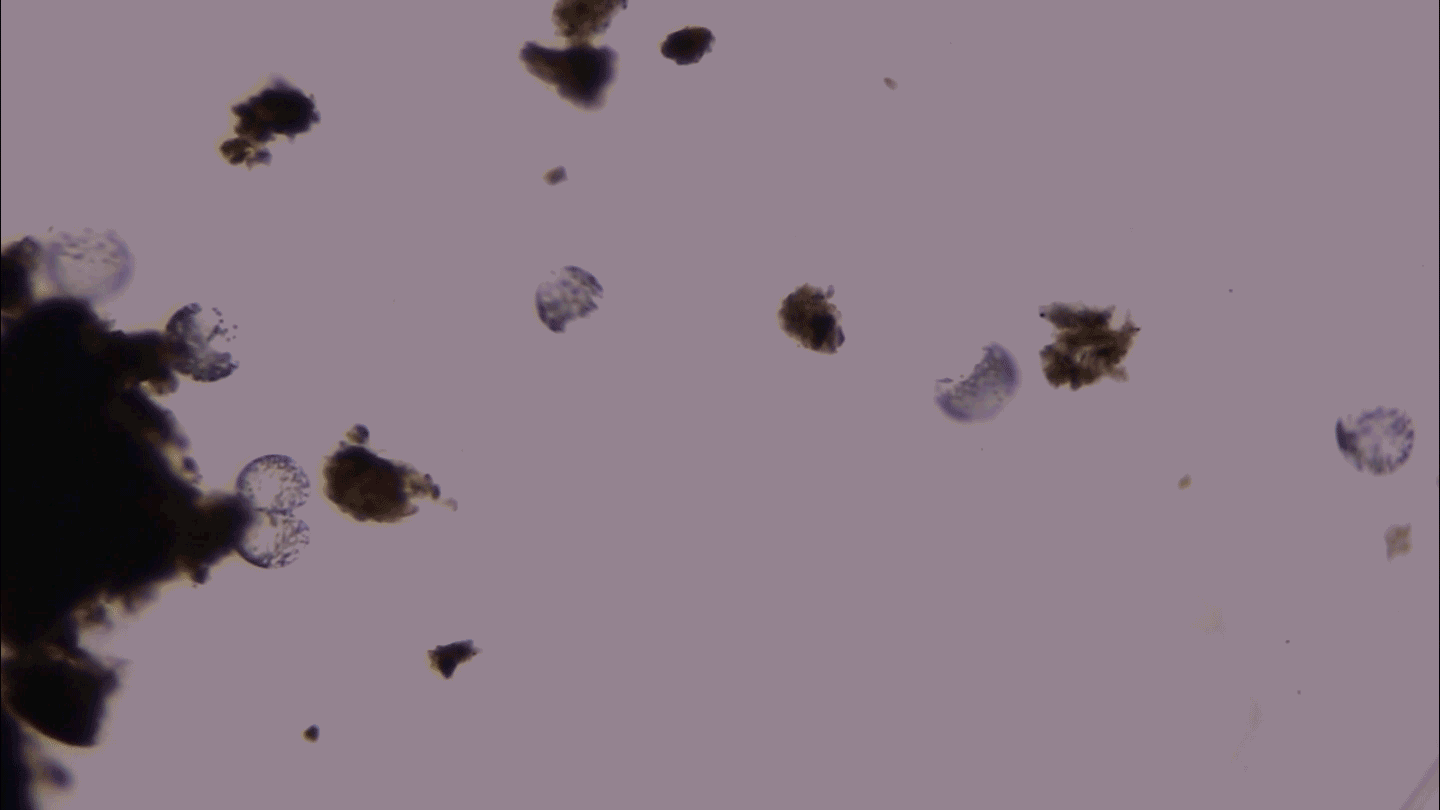
SHAPE SHIFTER Candida tropicalis usually grows as a harmless roundish budding yeast (green), but in the presence of two bacteria it stretches into long filaments (brown) that may provoke inflammation in intestines.
Djspring/wikimedia commons (CC BY-SA 3.0)
Fungi may affect gut health in unexpected ways, new research suggests.
High-fat diets may alter relationships between bacteria and fungi in mice’s intestines, contributing to obesity, researchers report October 11 in mSphere. In independent work, researchers report that a fungus teams up with two types of bacteria to fuel gut inflammation in people with Crohn’s disease. That work was summarized October 4 in Digestive and Liver Disease.
Together, the studies are part of a growing body of research indicating that relationships between the bacterial and fungal kingdoms can affect health, says David Andes, a fungal biologist at the University of Wisconsin School of Medicine and Public Health in Madison. Andes wasn’t involved in either study.
Scientists have already described links between health issues, including obesity, and gut bacteria — often called the microbiome. But far less is known about the role of the gut’s fungal mix, or mycobiome.
“To get the whole picture,” says Andes, “we’re going to need to start looking at the mycobiome in addition to the microbiome.”
As part of that picture, fungal biologist and pediatrician Cheryl Gale of the University of Minnesota in Minneapolis wanted to know whether high-fat diets change fungal communities as they do bacterial mixes.
Gale’s team fed mice either standard mouse chow or high-fat chow. As expected, mice on the high-fat diet gained weight, and the mix of bacteria in their guts shifted. Firmicutes bacteria associated with obesity increased, while Bacteroidetes bacteria decreased in abundance.
Fungi changed too. Mice fed high-fat chow had less Saccharomyces cerevisiae yeast and more Candida albicans in their guts than did mice that ate standard chow. S. cerevisiae is a yeast used in making wine, beer and bread and has been associated with good health. C. albicans is an organism that causes many yeast infections.
Gale’s team also discovered that relationships between bacteria and fungi changed when mice’s diets were changed. Her team can’t yet show a direct connection between the composition of gut fungi and obesity, but suspects that shifting interactions between bacteria and fungi might lead the host to gain weight.
The results are “very, very preliminary,” Andes says. “There are lots of theories to test.”
Meanwhile, other researchers are investigating how a microbial triad might contribute to an inflammatory bowel disease. People with Crohn’s disease have an overabundance of Candida tropicalis fungus along with Escherichia coli and Serratia marcescens bacteria, say Christopher Hager and Mahmoud Ghannoum of Case Western Reserve University School of Medicine in Cleveland.
When grown separately in lab dishes, the organisms “grew fine,” says Hager. “They formed nice little colonies. But when you mixed all three of them together, they just grew out of control. They form these intense, large robust biofilms.” Biofilms are structured microbial communities that can shield bacteria from antibiotics, making them hard to kill.
By itself C. tropicalis grows as a harmless budding yeast, Hager says. But in the presence of bacteria, the fungus stretches out into long filaments. Electron microscopy showed that E. coli fuse to the fungal growths. Meanwhile S. marcescens make protein strings that somehow stabilize the biofilm. That’s good news for the microbes: Their partnership allows them to outcompete loner bacteria and fungi.
But biofilms are bad news for the gut. Together the three organisms — but especially the fungus —may promote intestinal inflammation, a symptom of Crohn’s disease and other bowel disorders.
Hager and Ghannoum propose that giving Crohn’s disease patients antifungal drugs and then adding beneficial fungi, such as S. cerevisiae, could create a healthier microbe balance in the gut.
It’s still too early for doctors to change their patients’ treatments based on these studies, says Andes. But a full understanding of health should include all the kingdoms of life, he says.
Editor’s note: This story was updated November 9, 2017, to correct a general description of Crohn’s disease and to delete a reference to mice in describing how biofilms made of a fungus and two bacteria may promote inflammation.