Genetic editing can delete deleterious mitochondria
New approach avoids “three-parent” approach to treating DNA disorder
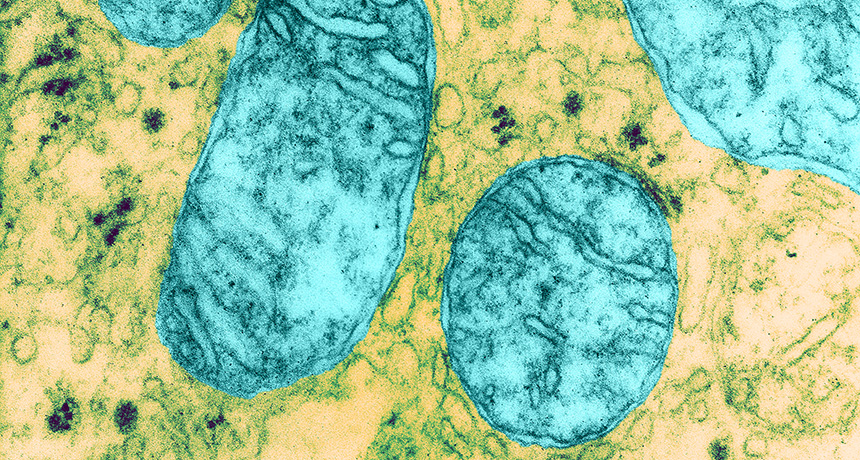
HEAVE HO Mitochondria (blue), energy-producing structures in cells, may get mutations that lead to diseases in people. Researchers have described a new genetic engineering technique that will eliminate mutant mitochondria from eggs and early embryos to prevent mitochondrial diseases from passing from mother to child.
Martin M. Rotker/Science Source
- More than 2 years ago
A new genetic engineering technique could help prevent mitochondrial diseases without the ethically sticky “three-parent problem” of another recently developed method.
The three-parent method involves transferring the nucleus of a prospective mother’s egg into a donor egg containing healthy mitochondria in order to replace mutant mitochondria with healthy ones.
The new gene-editing method, researchers report April 23 in Cell, removes or depletes the number of mutant mitochondria from eggs or early embryos. This approach could keep mitochondrial diseases from being passed from mother to child.
Mitochondrial depletion has advantages over the nuclear transfer technique, says Michio Hirano, a neurologist at Columbia University Medical Center who wasn’t involved in the study. “You don’t need an egg donor. You don’t need to involve a third party.”
Mitochondria are organelles that produce energy and perform other vital functions inside cells. The organelles have their own DNA. About one in 5,000 babies is born with a mutation in the mitochondrial DNA that can lead to diseases affecting energy-hungry organs, such as the brain, heart, muscles, liver and kidneys. There is currently no cure or effective treatment for these diseases.
“Instead of focusing on treatment, we’re focusing on prevention,” says molecular biologist Alejandro Ocampo of the Salk Institute for Biological Studies in La Jolla, Calif.
Together with reproductive biologist Pradeep Reddy, also at Salk, and other colleagues, Ocampo devised a method that targets DNA-cutting enzymes to unwanted mitochondria. The researchers experimented with mouse cells that carry two different types of normal mitochondria, known as BALB and NZB.
In the mouse experiments, the researchers designed molecules called TALENs that would cut DNA only in the NZB mitochondria. When the mitochondrial DNA is cut, it gets degraded. The cell then eliminates the damaged mitochondria, shifting the relative balance of mitochondria within the cell toward the desired type.
A similar experiment using a different type of DNA-cutting enzyme diced only the BALB mitochondrial DNA. Untreated mice had nearly 80 percent BALB mitochondria. But eggs or single-celled embryos treated with the enzymes produced healthy baby mice, with less than 20 percent BALB mitochondria. A shift of that magnitude would almost certainly eliminate mitochondrial diseases in humans, Ocampo and Reddy say.
The researchers also fused mouse egg cells with cells from patients with two types of mitochondrial diseases. TALENs directed specifically at the human mutant mitochondria reduced their number.
“The general concept is very exciting,” says Hirano.
This technique is simpler than mitochondrial replacement therapy, in which the nucleus of the prospective mother’s egg is transferred to a donor’s egg (SN: 11/17/12, p. 5). In vitro fertilization then creates an embryo that essentially has three parents: nuclear DNA from the mother and father and mitochondrial DNA from the egg donor. That technique was recently approved for clinical use in the United Kingdom.
Altering mitochondrial DNA doesn’t change a person’s looks, sex, intelligence or any other traits beyond energy production, Hirano says. That makes some people more comfortable with the ethics of genetically altering mitochondria than they are with changing genes in the nucleus, the compartment containing the rest of the genetic information for building a person.
Any technique that changes DNA in eggs or sperm — the germ line — produces changes that can be passed to future generations. Germline engineering is prohibited in 40 countries and some scientists have recently called for a moratorium on altering human germline DNA.
Mitochondrial depletion is still genetic engineering and may run afoul of ethical prohibitions about altering DNA that can be passed to subsequent generations, Hirano says. But Ocampo and Reddy say their technique is simpler and less ethically fraught than other germline editing techniques.
“We’re not trying to correct the mutation, we’re trying to eliminate it,” says Reddy. The technique leaves no altered DNA to pass to the next generation.
Eliminating faulty mitochondria may escape some definitions of germline editing, but toes up to the boundary of what is acceptable, says Marcy Darnovsky, executive director of the Center for Genetics and Society in Berkeley, Calif. It may open the door for genetic engineering of nuclear material.
And it may not be necessary to alter or replace faulty mitochondria to prevent diseases, she says. Eggs naturally vary in the amount of normal and mutant mitochondria they carry. Embryos created by in vitro fertilization could be tested before implanting into the uterus to determine which are likely to have the disease, Darnovsky says. “These seem like good arguments for not tiptoeing up to that line.”