Fluid in superdeep diamonds may be from some of Earth’s oldest unchanged material
Studying such a pristine piece of early Earth may yield insights about the planet’s formation

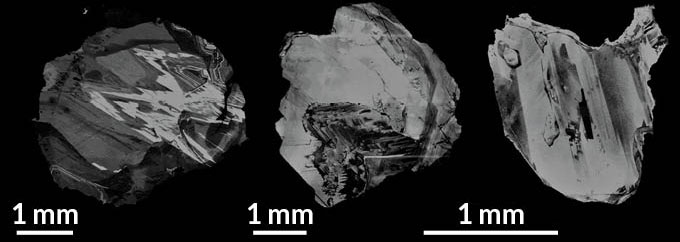
MANTLE SAMPLES Fluids trapped inside diamonds from Juina, Brazil, (pictured) bear the chemical signatures of primordial rock nearly as old as Earth itself.
Graham Pearson
- More than 2 years ago
A surprisingly hardy reservoir of rock left over from just after Earth’s formation still lurks deep inside the planet, according to a new analysis of superdeep diamonds.
Fluid trapped inside these diamonds, forged hundreds of kilometers underground in Earth’s mantle, bears the chemical signatures of rock that has remained relatively undisturbed for billions of years. This holdout of primordial rock may be nearly as ancient as Earth itself — making it some of the oldest preserved material on the planet today, researchers report in the Aug. 16 Science. Understanding the characteristics and preservation of such a pristine piece of early Earth may yield new insights into the formation and evolution of the planet.
Past chemical analyses of volcanic rock have hinted that Earth’s mantle might contain a repository of extremely old material. But scientists weren’t sure whether such an unspoiled relic could withstand the continual churning and mixing of material there. Evidence from volcanic rock is hard to trust on its own: Molten rock tends to get contaminated as it pushes up through the crust, and it’s difficult to pinpoint where specific bits of rock originated, says Suzette Timmerman, a geochemist at the Australian National University in Acton.
For a more direct glimpse into Earth’s interior, Timmerman and colleagues scrutinized 24 superdeep diamonds from Brazil that were known to have formed 410 to 660 kilometers underground. As the diamonds crystallized, they swallowed up microscopic pouches of fluid from their surroundings (SN Online: 3/8/18). When a superdeep diamond rises to Earth’s surface, its sturdy crystal structure shields these diamond inclusions from contamination, Timmerman says. “It’s exactly preserving the chemical composition at those really deep depths.”
Using mass spectrometry, the researchers cataloged different isotopes, or types, of elements enclosed in superdeep diamond inclusions. “It’s quite impressive how they’ve managed to analyze a signal which must be very tiny” from fluid bubbles less than a micrometer across, says Andrew Thomson, a geologist at University College London.
Timmerman’s team compared the abundance of two helium isotopes, helium-3 and helium-4, in the diamond inclusions. Unlike helium-4, Earth has not generated any new helium-3 since its formation, and any helium-3 that reaches Earth’s surface escapes into space, Thomson says. So material that is relatively rich in helium-3 compared with helium-4 must have formed early in Earth’s existence and been isolated for a very long time from the surface — and any other internal processes that could siphon away helium.
In the superdeep diamond inclusions that were richest in helium-3, the ratio of helium-3 to helium-4 was about 1-to-14,300. That may not seem like much, but it’s about 50 times higher than the ratio of helium-3 to helium-4 ratio seen in air: just 1-to-about 714,000.
This suggests the fluid inclusions date back to around the time Earth formed, some 4.5 billion years ago. Based on the depths where the diamonds crystallized, this reservoir is likely at least 410 kilometers underground, but it’s unclear how much deeper it might sit. Determining the reservoir’s exact location may help explain how it formed and remained undisturbed for so long, Thomson says.
The overall chemical composition of the reservoir is still a mystery, “but it must be quite dense in order to avoid mixing with the rest of the mantle,” says Timmerman, whose team will present the work at the Goldschmidt geochemistry conference in Barcelona on August 23. Examining other isotopes encased in superdeep diamond inclusions, like metals, may offer more clues as to the chemical blend inside this reservoir.
Another lingering question is whether the reservoir comprises one giant mass, or multiple smaller pockets of age-old material. Seismic observations have uncovered a pair of especially dense globs of rock at the base of the mantle (SN: 6/11/16, p. 13), “but we don’t know for sure whether it’s this reservoir or something else,” Timmerman says.







