- More than 2 years ago
Normal 0 false false false MicrosoftInternetExplorer4 By identifying a new way to wrestle fluorine from carbon compounds, chemists may now be able to break down certain types of greenhouse gases before they reach the atmosphere.
Some of the most potent manmade greenhouse gases are also among the most difficult chemicals to destroy, and the most persistent once released into the environment. Scientists report in the Aug. 29 Science that they have managed to force these gases to chemically react, turning them into more benign compounds.
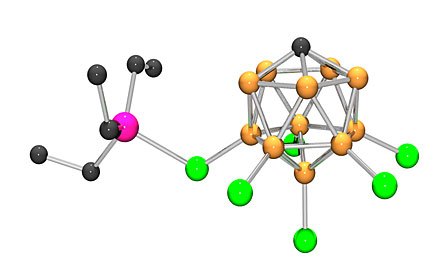
For now, the process offers only a proof of concept. But the work opens the door to new ways of disposing of these materials. “Our process allows us to take a hydrofluorocarbon and transform it so that it’s no longer a greenhouse gas,” says study coauthor Oleg Ozerov of BrandeisUniversity in Waltham, Mass.
“It’s a very hard nut to crack, and they finally got it to work,” says Robert Crabtree of YaleUniversity.
Fluorocarbons, compounds in which carbon and fluorine atoms are bonded together, are a wide class of chemicals, with applications ranging from anesthetics to nonstick pans. Those in a class called hydrofluorocarbons, or HFCs, have been used as refrigeration fluids to replace chlorofluorocarbons, which were implicated in the thinning of the ozone layer.
Ironically, though, HFCs have been found to have a different drawback: They trap heat extremely well. Once released in the atmosphere, HFCs contribute to the greenhouse effect and to global warming — which made them a target of the Kyoto Protocol, the 1997 international treaty aimed at preventing climate change.
Getting rid of HFCs is difficult, because carbon-fluorine bonds are notoriously resistant to change, or, chemically speaking, inert. That means HFCs are hard to break up through reactions with other substances. Fluorocarbons do react with some potent acids, but those tend to be expensive or they only work at very high temperatures.
But Ozerov and Christos Douvris, now at the University of Colorado at Boulder, have found a way to use acids as reusable catalysts, which don’t get consumed while they help HFCs react chemically. This reaction also has the advantage of working at room temperature.
Ozerov and Douvris found out that a powerful acid, when used in organic solvents such as benzene, forces HFCs to react chemically with silanes, silicon-hydrogen compounds, leading the HFCs to swap out their fluorine atoms with hydrogen atoms. The resulting compounds are then easier for chemists to deal with, Ozerov says.
Crabtree says that the chemical industry may now be able to find economical ways to dispose of existing stockpiles of HFCs, in the expectation that these chemicals will be banned sooner or later. “All of these persistent materials risk being forbidden at some point in time,” he says.
Meanwhile, Reyes Sierra of the University of Arizona in Tucson points out that the team’s approach, while potentially useful, would not help clean up fluorocarbons already in the environment, for example in aquifers. The catalyzing action of the acids — part of a class of compounds called Lewis acids — would be blunted in the presence of water, and it would be impractical to separate the pollutants from the water. Still, Sierra calls the team’s results a “major scientific advance.”
In Environmental Science & Technology, Sierra and her collaborators recently described how another ubiquitous class of fluorocarbons called PFOSs break down in water in the presence of vitamin B-12. PFOSs are not greenhouse gases, but they have been found to be toxic.