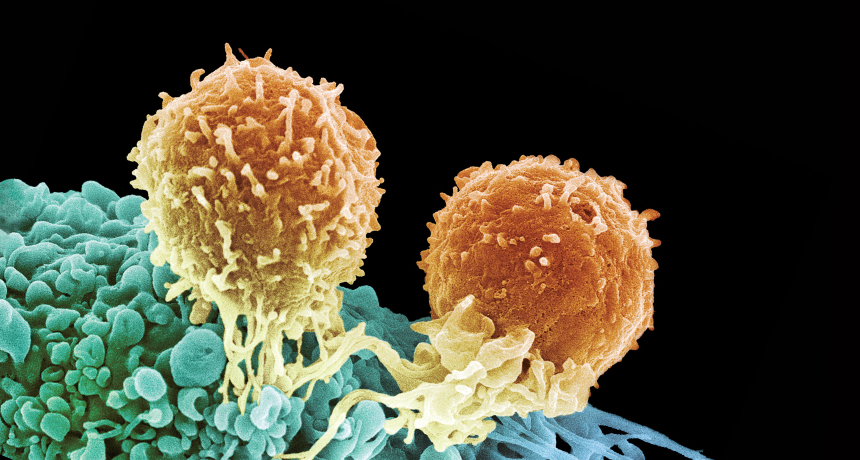
T-FORCE Two T cells (orange) attack a cancer cell (blue), using special receptors to zero in on the cancer. Immunotherapies are boosting the potency of T cells.
Steve Gschmeissner/Science Source
Doug Olson had all but lost his 14-year battle with leukemia. Exhausted and weakened by chemotherapy, his body no longer responded to any of the handful of drug treatments he had been given over the years. In 2010, his doctors suggested a different strategy: beefing up the disease-fighting immune cells in his body.
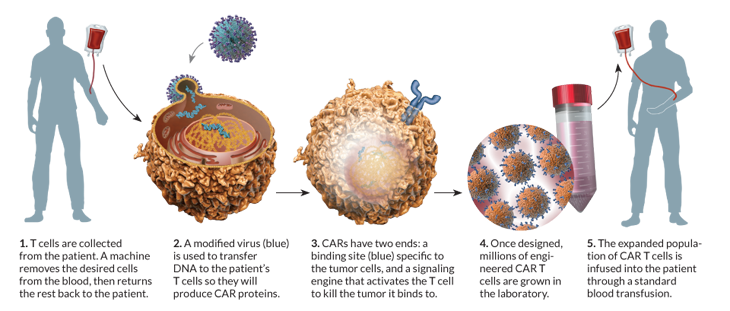
Researchers picked out certain immune cells from Olson’s blood, then inserted a virus into the cells. The virus provided new genes that prompt the cells, known as T cells, to attack leukemia cells. When the altered T cells were delivered back into Olson’s veins, his immune system became a cancer-seeking weapon.
One month after treatment, Olson was in complete remission. His doctors could find no sign of cancer in his blood or bone marrow. Today, at age 67, he remains cancer-free. Recently retired, Olson says he no longer worries about how long his remission will last; he’s taken up sailing and running half-marathons.
Other cancer patients — children and adults — have seen similar results with reprogrammed immune cells. At least six research groups have treated more than 100 patients with leukemia and other blood cancers by using the patients’ own enhanced T cells.
“It won’t be long, I think, before the first line of therapy for some types of cancer won’t be chemotherapy, it will be immunotherapy,” says Carl June of the University of Pennsylvania School of Medicine in Philadelphia, one of the developers of T cell–boosting therapies.These treatments are part of a medical strategy that researchers have been working on for decades, and their efforts are finally beginning to pay off. There are now dozens of early human trials around the world using engineered immune cells, and the pharmaceutical industry is beginning to invest.
Researchers are using refurbished T cells with two main goals: to encourage the immune system to attack bad players — cancers or viruses — or to tamp down the immune system so it will stop attacking the body’s healthy tissues, as it does in some autoimmune diseases such as type 1 diabetes and rheumatoid arthritis. Though several obstacles remain, scientists are cautiously hopeful.
“Though there’s still work to be done to make these treatments safe and effective, I’m optimistic now that there will be a role for cells as drugs,” says immunologist Jeff Bluestone of the University of California, San Francisco.
Finding ways to intervene in the immune system has not been easy. The human immune system is a busy network of organs and cells that attempt to keep harmful bacteria, viruses and parasites out of the body, and to destroy agents that manage to invade. This system includes an army of white blood cells that patrol the body, looking for trouble.
Certain white blood cells called macrophages are constantly on guard, tasked with destroying any germs they encounter. If an infection sets in, a more powerful force of white cells, namely B cells and T cells, joins the assault. B cells secrete substances to identify and neutralize invaders. Some T cells fight pathogens directly and some activate other cells to fight.
Killer T cells, one of several T cell types, are one of the immune system’s most powerful weapons. They connect directly to infected cells and tumors, secreting chemicals to kill them. To identify its target, a T cell relies on receptors that sit poised on its surface. These receptors recognize and bind to surface structures, called antigens, found on pathogens.
Enhanced recognition
When all goes well, this recognition system allows T cells to remove infected cells and tumors. But occasionally, a cell develops traits that stymie immune surveillance. Some cancers, for example, have mutations that cloak them from patrolling immune cells. HIV and other viruses evolve to evade normal immune responses as well.
Scientists reasoned that adding certain genes to T cells might help them fight those evaders. The right gene added could instruct a T cell to make a receptor that zeroes in on an antigen found only on cancer cells, for example.
Although early attempts succeeded in getting genes into cells, the T cells soon exhausted themselves when placed inside a living host. In many cases, the engineered cells couldn’t divide to make new cells.
June and his colleagues found a way to transform the T cells into more viable weapons, using a disabled form of HIV to deliver the genes into the T cells’ DNA. Once inside, the genes produce a protein called chimeric antigen receptor, or CAR, which enables the T cell to recognize and specifically kill certain cancer cells.
June designed his CAR T cells to carry receptors for an antigen called CD-19, found on the surface of some leukemia cells. Once altered, the T cells seek and destroy cells that carry CD-19. Those altered cells continue to divide for several weeks, boosting the CAR T cell population. What’s more, CAR Ts live longer than normal killer T cells — they can attack again and again.
“Essentially, they become serial killer cells,” June says. “The cells kill a target and then go kill another one.”
In 2011, June’s group showed that after treatment, some CAR T cells become memory T cells, remaining primed to initiate attacks if the cancer tries to recur. “That’s very different from standard drugs, which exert an effect and then are eliminated from the body,” June says.
June’s team has used CAR T cells to treat 62 patients with late-stage leukemia, including 32 with chronic lymphocytic leukemia, or CLL, and 30 — five adults and 25 children — with acute lymphoblastic leukemia. Of the 30 patients with ALL, 27 had complete remissions, all signs of cancer disappeared, he says.
CLL mainly affects adults and is not curable without a bone marrow transplant (BMT). Instead of getting a BMT, Olson chose to be one of the first three patients to receive CAR T treatment for CLL in 2010. For Olson and one other patient, the cancer disappeared, June’s group reported in 2011 in Science Translational Medicine.
June’s team has had recent success engineering T cells against HIV as well. They programmed the T cells of 12 HIV-infected patients to make their bodies resistant to the virus. In one patient, blood levels of HIV became undetectable. That study, in collaboration with scientists at the Albert Einstein College of Medicine in New York City and California-based Sangamo BioSciences, was published in the New England Journal of Medicine in March. (Article continues below graphic)
CAR Ts in the fast lane
June and other researchers have begun early-stage pilot trials using CAR T cells against pancreatic, breast and brain cancers and melanoma at centers across the country, including Memorial Sloan-Kettering Cancer Center in New York City, the Fred Hutchinson Cancer Research Center in Seattle, and Baylor College of Medicine in Houston.
Large-scale studies have not yet happened, but pharmaceutical companies already are making plans to take immune-cell therapies to market. Novartis is working with June to develop his CAR T cells. “I’ve told the team that resources are not an issue. Speed is the issue,” Novartis Chief Executive Joseph Jimenez told Forbes magazine in May. And researchers from Fred Hutchinson, Memorial Sloan-Kettering and Seattle Children’s Research Institute launched Juno Therapeutics in Seattle to bring their CAR Ts to market.
Although studies to date suggest that CAR T cells are safe for most patients, some patients experience dangerously high fevers, nausea and chills, caused by chemicals called cytokines that pour out of T cells when the immune system becomes highly activated. And CAR T cells can kill unintended cells, such as B cells, which also carry the CD-19 antigen. This side effect continues long after the treatment has been stopped. Olson, for instance, visits his doctor several times a year to get an injection of immune globulin, to replace the proteins that B cells make.
To counteract such side effects, future versions of CAR T cells may be equipped with control circuits so that doctors can activate or deactivate them as needed, June says.
Other researchers are devising ways to focus the immune system on tumors with difficult-to-recognize antigens. Steven A. Rosenberg of the National Cancer Institute, an early developer of immunotherapies for cancer, is directing immune cells to zero in on other targets found in cancer cells but not on normal cells.
When normal cells become cancerous they acquire a number of mutations, and those mutations are unique to each patient. In a paper published May 9 in Science, Rosenberg’s team describes how they sequenced the genome of a patient’s cancer cells, then identified immune cells that could target a specific mutation on that patient’s tumor, which had spread from the bile duct to the liver and lungs. Extracting these immune cells from the patient’s tumor and growing armies of them in the lab, the scientists infused the cells back into the patient, and her tumors shrank. Though the treatment didn’t eliminate the tumor, Rosenberg says it’s a “proof of principle” that you can identify unique mutations in a tumor and then direct immune cells to recognize and destroy the cancer.
“It’s a blueprint for identifying and targeting a single amino acid change among all of the proteins present in that cancer,” he says.
While still in early development, Rosenberg’s findings open up possibilities of targeting many common cancers with patients’ own immune cells. These cancers originate in epithelial cells that line the body’s organs. Rosenberg says such cancers frequently occur in the breast, colon, liver, pancreas and esophagus, and often spread to other parts of the body.
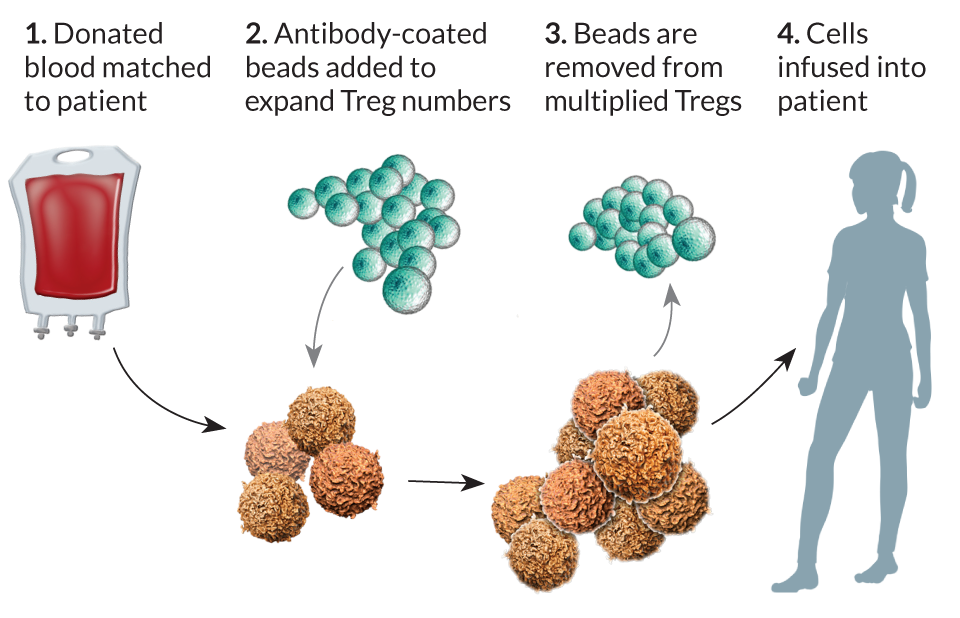
Immunotherapy against cancer and viruses is about stimulating the right T cells to act. But sometimes the immune system gets overstimulated and attacks the body, or in the case of organ transplants, the body attacks the donated organ. Scientists are designing ways to steer the immune cells to fight only enemies and ignore the body’s own tissues. Special T cells called regulatory T cells, or Tregs, help police the immune response, keeping overzealous killer T cells in check.
Autoimmune diseases too
Tregs suppress excessive immune responses. Finding ways to alter Tregs would be helpful in treating autoimmune diseases such as rheumatoid arthritis or type 1 diabetes. It would also be useful in cases of organ transplant, says UC San Francisco’s Bluestone. His lab is developing ways to use specific Tregs in transplant settings so that the cells recognize the transplanted organ and “educate” the immune system to see the organ as part of its own tissue. Such treatments may eventually eliminate the need for ongoing treatment with immunosuppressive drugs.
Current drugs used to suppress the immune system may work for years, but many transplanted organs are ultimately rejected by the patient’s body.
To retrain the body’s peacekeeping force, Bluestone’s group focuses on a population of cells called Foxp3–expressing Tregs. These cells are master regulators of the immune system, Bluestone says.Foxp3 Tregs squelch the action of other T cells directly, but are also appealing because of a two-pronged trait called bystander suppression. First, the cells create an overall suppressive environment in areas where they do their work. As a result, immune cells that recognize a foreign antigen such as a transplanted organ learn to make peace with the foreigner. Second, the Foxp3 Tregs convert other cells in the area into peacekeepers.
“You end up with this situation where you not only suppress locally, but you turn other cells into regulatory cells that perpetuate and amplify the effect of the Tregs,” Bluestone says.
Still, he says, it’s unlikely that a simple infusion of Tregs will work as a stand-alone therapy in transplants, at least for now. For starters, Tregs are often outnumbered by other cell types. After a transplant, a mix of several T cell types, called effector cells, attack and kill new organs and expand into armies of millions, making it harder for the Tregs to suppress.
Bluestone’s group has found ways to combine the Foxp3 Tregs with drugs to temporarily deplete a large number of effector cells. That gives the Tregs a chance to take over. It’s that combination, Bluestone says, that will make Tregs a useful therapeutic tool.
“It’s a balancing act,” he says. “The more effector cells you have, the more Tregs you need to suppress them. Anything we can do to reduce the number of effector cells allows the Tregs to be more effective.”
University of Minnesota oncologist Bruce Blazar uses an approach similar to June’s and Rosenberg’s to increase Treg numbers: Pull the cells out of blood and expand their numbers before putting them into patients. The replication process ups the number of Treg cells by tens of millions in several weeks. Blazar is using this approach to give patients a better chance for a successful bone marrow or organ transplant.
The technique has shown promise in treating graft-versus-host disease, a complication of some bone marrow transplants in which T cells from donor bone marrow perceive a recipient’s body as foreign and attack it. An early trial suggests that the technique can ameliorate some damaging effects of the disease. Blazar reported his results in February at the American Association for the Advancement of Science annual meeting.
Clinical trials also are under way to test Treg cells against autoimmune disorders. Bluestone’s group is treating patients with type 1 diabetes with infusions of Tregs and plans to test the treatment in patients with lupus next year. Scientists at Dana-Farber Cancer Institute in Boston showed in 2013 that infusions of Tregs successfully blocked the development of a rheumatoid arthritis-like condition in mice.
Still, challenges remain. Not all patients respond to treatment with engineered T cells. Bluestone says scientists also have not yet found ways to control the enhanced T cells should they become overly active or overly suppressive. Researchers are still working on genetic and biochemical approaches to eliminate the engineered cells if they cause unwanted side effects, or find ways to adjust them if they begin working in ways they should not.
In most cases, the goal is to direct Tregs to sites where they’re needed, rather than having them working systemically, Bluestone says.
Eventually, he adds, researchers might find ways to genetically modify the cells, following the example of engineered T cells for cancer. Though it will probably take years to get to that point, he says the field of immunotherapy is now exploding, and trials are moving rapidly in many areas.
“It’s no longer early days for cell therapy,” Bluestone says.
June agrees: “The issue now is not does cell therapy work, but how can it become available everywhere, and not just at boutique cancer centers.”
He suggests taking a cue from manufacturing methods used by the automotive industry.
“Cars in Detroit are now made mostly by robots,” he says. “We need to find ways to make our CARs — our T cell cultures — in an automated way, or else it won’t happen on a large scale.”
Editor’s note: This article appears in the June 14, 2014 Science News under the headline, “T-Force: Reprogrammed immune cells emerge as weapons in the body’s disease-fighting arsenal.”