- More than 2 years ago
Gene therapy has been on a roller coaster in recent years. Experiments in adding genes directly to patients’ cells have shown promising signs, but the technical and clinical momentum has been drained repeatedly by bad results. These include the death of a young man in 1999 and two recent cases of cancer in children. The clinical trials in these instances used inactivated viruses as vectors to shuttle genes into patients’ cells. Scientists hold those viruses to be partly to blame for the devastating outcomes. So, many researchers are focusing attention on ways to introduce DNA into a patient without using a virus.

For 2 decades, away from the noise of the latest ups and downs for viral vectors, chemists and materials scientists have been doggedly investigating and improving on other strategies such as using capsules that protect and guide DNA into cells and methods of introducing naked DNA. Though these techniques remain works in progress, they may eventually present a safer alternative to viral transporters of DNA.
Virus trouble
Natural viruses can target and deliver genetic material with the utmost efficiency. That’s why most gene therapy trials have employed them as vectors. However, even inactivated viruses pose certain risks.
In the study at the University of Pennsylvania in Philadelphia that led to the 1999 death of 18-year-old Jesse Gelsinger, researchers were testing the safety of a new version of adenovirus, a type of natural virus that usually causes respiratory infections. Gelsinger had a nonfatal deficiency in a liver enzyme that removes ammonia from the blood. The scientists gave Gelsinger adenovirus containing therapeutic DNA, but he developed a massive immune response to the virus and died just days after the injection.
In the cases of the children who developed cancer, French scientists were using a virus known as a retrovirus to put healthy genes into 11 children suffering from severe combined immunodeficiency syndrome. Children with this disease contract infections easily, and many die before their first birthday. The genetic disease is sometimes called bubble-boy syndrome because a boy born in 1971 survived 12 years by living within a protective bubble.
Retroviruses seem promising as vectors because they’re efficient at getting genes into cells and the human immune system doesn’t usually react strongly to them. But experiments have shown that these viruses sometimes incorporate new DNA into a cell in deleterious ways. Researchers suspect that such a viral mistake led to leukemia in one 3-year-old boy, who was diagnosed with the cancer in September. Then last week, the U.S. Food and Drug Administration placed about 30 trials using retrovirus vectors on hold after a second child in the French trial developed a leukemia-like condition.
Both children are now being treated with chemotherapy.
Despite these problems, many researchers are optimistic that someday they’ll develop a genuinely safe viral vector. For that reason, investigators are devising new viral vectors and tweaking old ones to create agents that will efficiently deliver genetic material without triggering immune attacks or other deadly side effects.
Better fakers
While some scientists are working to improve carriers based on viruses, others are betting on a nonviral option. Boosting the strengths of synthetic vectors–while ridding them of their weaknesses–is the goal of many laboratories.
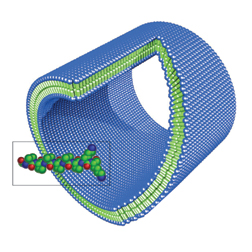
Chemists and materials scientists have been creating virus-size structures to temporarily encase and protect genetic material, diffuse nimbly through three-dimensional tissue, zero in on target cells, enter those cells, and then release genetic cargoes at the proper locations. “What we’re all trying to do is recapitulate the properties of a small virus,” says Francis Szoka of the University of California, San Francisco.
Szoka has been investigating nonviral gene–delivery methods since 1978. He works with liposomes, which are layers of lipids that assemble into vesicles 100 nanometers or so in diameter, or about the size of a big virus.
Recently, Szoka has been developing vesicles composed of both lipids and polymers.
These 70-nm-wide structures can be loaded with DNA and are sensitive to the acidity, or pH, around them. If injected into the bloodstream of test animals, they can circulate intact at the near-neutral blood pH of 7.4, he says. However, once they enter a cell, the acidity in the uptake compartment increases to a pH of 5 to 6 and the vesicles fall apart, releasing their genetic loads into the cell’s cytoplasm.
From there, the delivered genes make their way to the nucleus, where they can initiate production of specific proteins.
When Szoka and his colleagues tested their vectors in animal cells growing in laboratory dishes, the vesicles were efficient at carrying genetic material into cells. Beyond that, the inserted genes then directed the cells to make the proteins they encode, Szoka says.
In experiments on mice, toxicity doesn’t seem to be a problem. The vectors seem to go primarily to the liver, which naturally removes particles of this size from the animal. Szoka suggests that the best use for his potential vectors will be for treating genetic diseases, especially those of the liver.
Mark Davis of the California Institute of Technology in Pasadena is taking another approach to virusfree gene delivery. With the goal of developing treatments for cancers that have spread from their tissue of origin, he and his students design nanoscale structures made of polymers.
Previously, most polymers used in new vectors had been derived from materials already made for other applications, Davis says. When researchers have adapted these substances for gene delivery, they’ve often been too toxic. They also haven’t proven as effective as viruses at entering cells.
For now, it’s a tradeoff, at best: Exchange the risk posed by viruses for potentially safer synthetic substitutes, but lose nature’s efficiency. “There’s always a balance between the good and the bad,” says Jean-Paul Behr of the Université Louis Pasteur–Strasbourg in Illkirch, France.
If scientists used a vector made from a polymer specifically designed to have low toxicity, they might be able to administer multiple doses of gene-bearing treatments and thereby overcome the vectors’ relative inefficiency, Davis suggests.
Toward that end, he and his coworkers are designing nanoscale delivery vehicles made primarily from molecules called b-cyclodextrins, which have been used successfully in other biological systems. The vectors will need to survive in patients’ bloodstreams and dispense genetic material after they enter a target cell.
Davis calls his 100-nm-diameter spheres “smart nanoparticles.” By changing the chemistry of their surface, Davis says, he can direct them to different types of cells.
At an American Chemical Society conference on drug delivery last October in Boston, Davis reported that these particles did go to the targeted cells in mice. In one experiment, the goal was mouse liver cells; in another, it was human prostate-tumor cells transplanted into mice. The DNA carried by the polymer vectors directed the mouse cells to make specific proteins.
Many other researchers use genetic material that, when successfully assimilated into targeted mouse cells, produces an easily identifiable marker protein. Davis’ nanoparticle system delivered genes that could produce both a marker protein and a therapeutic protein, such as the tumor suppressor p53. Davis’ colleague described this work, which is being commercialized, at a meeting in San Diego on Dec. 10, 2002.
In a vector, it’s useful to have several components, each one performing a special function, says Behr. For example, one molecule might condense DNA into a tight package for delivery to a cell. Others would bind to receptors on specific cells.
Adding certain protein fragments, or peptides, to the DNA would help it enter the nucleus of a cell. Behr and his colleagues are exploring such methods.
Polymers are particularly versatile for designing nonviral vectors, says Robert Langer of the Massachusetts Institute of Technology (MIT). However, creating the best one for delivering genes to a specific bodily location is, in his words, “a real engineering challenge.”
In an effort to speed the discovery of effective vectors, Langer and his coworkers have now developed a way to rapidly screen the toxicity and efficiency of 2,400 structurally different polymers at once. The team has examined varieties of biodegradable polymers known as poly(b-amino esters).
Several polymers that made it through the screenings look particularly promising, and those will be tested in animals, says Langer.
Shuguang Zhang, also of MIT, is looking at even smaller elements in his approach to nonviral-vector discovery. By choosing and linking specific sequences of amino acid, he and his coworkers have created short peptides that have a positively charged end made of the amino acids lysine or histidine. When placed in a solution with DNA, these synthetic protein fragments assemble themselves into nanoscale capsules and tubes containing DNA.
When Zhang tested these molecular devices, they successfully delivered their DNA to at least some cells growing on laboratory plates. Zhang detected marker proteins encoded by the inserted DNA in 5 to 10 percent of the cells. Other labs are in the process of testing Zhang’s vectors in animals.
Although he’s still far from his goal of inserting working genes into 50 percent of the test cells, Zhang views these and other self-assembling peptides as promising.
Naked DNA
If viral vectors are risky and nonviral vectors are difficult to develop, why not get rid of the carrier altogether? Consider naked DNA.
If scientists could introduce pure DNA into target cells and tissues without encasing it in a virus, a polymer, or other material, they would eliminate concerns about the toxicity of those substances.
One of the earliest naked-DNA approaches coated tiny gold particles with DNA and then shot them through skin with a gunlike device. In another method, called electroporation, researchers send pulses of high-voltage current through tissue while simultaneously injecting solutions of DNA.
“Naked DNA is great, but it needs help,” says Leaf Huang of the University of Pittsburgh, who is also pursuing this approach, as well as lipid and polymer-based vectors. For one thing, he notes, unprotected DNA typically degrades in the body before it gets to its target.
Huang and his Pittsburgh colleague Feng Liu recently modified the electroporation process so that a metal syringe can double as the electrode. In the March 2002 Molecular Therapy, Huang and Liu reported that this technique is just as efficient as those using independent electrodes at much higher electric field strengths. Therefore, this new approach minimizes tissue damage.
In mouse studies, cells that received the naked DNA produced a marker protein for a day or two before the animals were killed and examined.
In another, decidedly less conventional experiment, reported in the June 2002 Hepatology, Huang and Liu found that they could induce mice to take injected DNA into their cells by massaging the animals’ abdomens. “Nothing can be as noninvasive,” says Huang. The physical pressure of this stimulation probably generates small pores on cell membranes that allow entry of DNA, Huang suggests.
Whether it’s viral, nonviral, or vectorless, all methods of gene delivery have a long way to go before becoming part of routine therapies, and scientists agree that there probably won’t be one best way to deliver genetic material for all of the diseases that gene therapy might treat.
But the difficult work that will be required to build these vectors is crucial to medicine. Says Davis: “It’s the delivery problem that’s holding back the field.”
****************
If you have a comment on this article that you would like considered for publication in Science News, please send it to editors@sciencenews.org.
To subscribe to Science News (print), go to https://www.kable.com/pub/scnw/subServices.asp.
To sign up for the free weekly e-LETTER from Science News, go to http://www.sciencenews.org/subscribe_form.asp.