Why the coronavirus’s delta variant dominated 2021
Delta’s unique constellation of mutations explains why it has wreaked so much havoc

Mutations to the delta variant of the coronavirus allow it to efficiently invade cells and gear up new attacks, as seen in this illustration of the virus’s life cycle.
Falconieri Visuals
By Tina Hesman Saey and Erin Garcia de Jesús
- More than 2 years ago
2021 was a year of coronavirus variants.
Alpha and beta kicked off the year, and several worrisome variants later, omicron is closing it out. How omicron may come to define the pandemic’s future remains uncertain. But even as omicron comes on strong, one variant, which rose to global dominance midyear in a way variants like alpha and beta never did, continues to largely define the pandemic right now: delta.
Things had actually seemed to be looking up in some parts of the world in the late spring and early summer of 2021, a year and a half into the COVID-19 pandemic. In the United States, for instance, millions of people were vaccinated, cases of the disease were falling, and people were beginning to socialize and resume normal activities.
But then delta hit hard. First spotted in India in October 2020, this variant of SARS-CoV-2, the coronavirus that causes COVID-19, quickly swept around the world, supplanting other versions of the virus in 2021 (SN: 7/2/21). Delta overwhelmed health care systems, tore through unvaccinated populations and showed that even the vaccinated were vulnerable, causing some breakthrough cases.
It soon became clear why delta wreaks so much havoc. People infected with delta make more of the virus and spread it for longer than people infected with other variants, researchers reported in Clinical Infectious Diseases in August. As a result, delta infections are more contagious. Consider two scenarios in a community where no one has immunity to the coronavirus: A person infected with an earlier version of the virus — the one first identified in Wuhan, China, that set off the pandemic — might spread it to two or three others. But a person infected with delta may transmit it to five or six people.
Delta owes its success to mutations in some of its proteins. Take, for instance, a mutation called R203M in the coronavirus’s nucleocapsid, or N protein, located inside the virus. This mutation may increase the amount of viral RNA that can be made or make it easier for the N protein to do its job, packing RNA into newly assembled viral particles, researchers reported in Science in November.
Sign up for our newsletter
We summarize the week's scientific breakthroughs every Thursday.
Mutations similar to delta’s have appeared here and there in other variants that proved themselves capable of spreading more easily or better evading the body’s immune defenses than the original virus. That includes alpha, first spotted in the United Kingdom; beta, first characterized in South Africa; and gamma, first noted in Brazil. The recently discovered omicron variant, first described in South Africa and Botswana, also shares some of the same mutations (SN: 12/1/21).
Some of delta’s grab bag of mutations are identical to those found in other variants, while others change the same protein building block, or amino acid, in a different way or pop up in the same part of the virus. For instance, alpha and omicron also have the same mutation of the 203rd amino acid in the N protein, but it is a different amino acid change than seen in delta. And some mutations are entirely new to delta.
Delta’s defining mutations
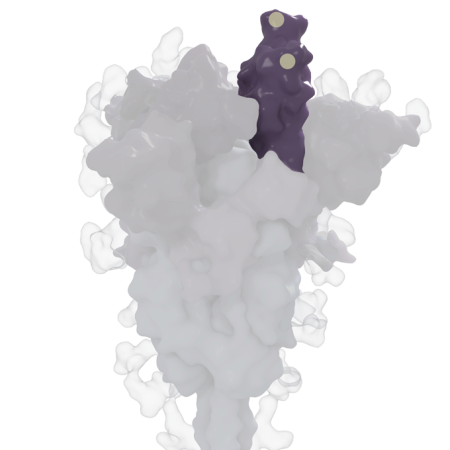
These mutations on the coronavirus’ spike protein are what define delta as delta. The spike protein helps the coronavirus attach to and enter human cells. The delta variant’s version carries a unique collection of mutations, marked by yellow dots in this 3-D rendering. Some of these mutations may help the virus more easily infect cells or hide from antibodies.
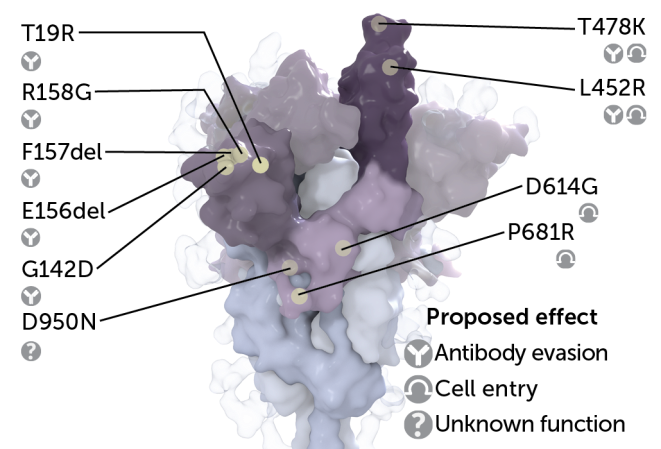
Scientists don’t yet know the effect that all those changes have on delta’s ability to replicate or spread to others. What’s more, delta continues to evolve, picking up additional changes over time. But studies have zeroed in on the unique constellation of mutations decorating the virus’ spike protein. It’s the knobby protein studding each coronavirus that helps the virus latch onto and invade human cells. What looks like an individual knob is in fact composed of three identical pieces that fit together, each carrying the same set of mutations.
Some of delta’s spike protein mutations may help the virus more easily break into cells, where it turns cell machinery into virus-making factories. Two of those, dubbed T478K and L452R, are advantageously located on the receptor-binding domain. This is the part of the spike protein that attaches to ACE2, a protein on the surface of host cells.
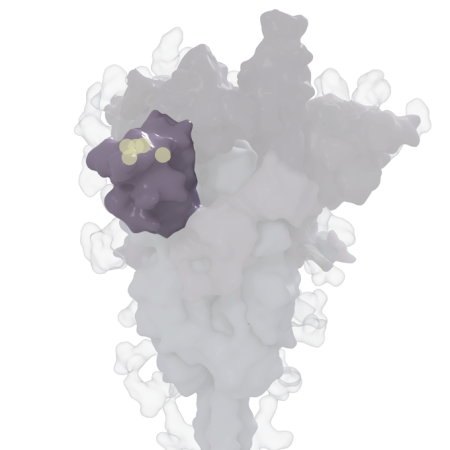
Other mutations show up in a region of the spike protein called the N-terminal domain, which is a known target of the immune system’s neutralizing antibodies. These mutations may help the virus evade those antibodies, which can stop the virus from infecting cells.
And yet two other mutations, P681R and D614G, may help prep newly made viruses to go out and conquer. Those mutations are nestled near the dividing line for two parts of the spike protein, S1 and S2. Those parts need to be split apart to allow the coronavirus to engage in the gymnastics needed to help it fuse with the membrane of its prospective human host cell.
Key regions of the coronavirus spike protein
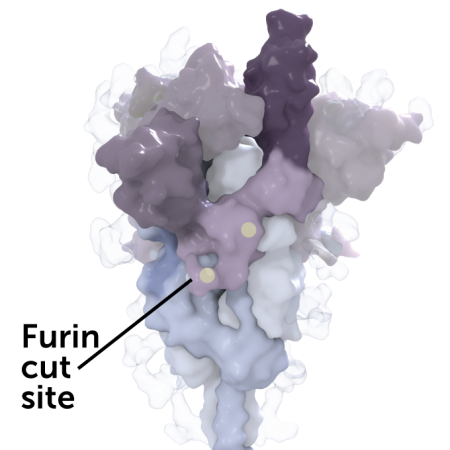
Human cells actually aid in this process: Inside infected cells a human protein called furin nicks the spike protein between the S1 and S2 segments, opening the receptor-binding domain so it can better grab ACE2. The P681R and D614G mutations may make the spike protein easier for furin to cut. Once snipped, newly-made viruses are primed to infect other cells.
Taken together, these mutations help delta break into cells more quickly and perform several tasks better than other variants do. As a result, in 2021, delta was able to become the dominant variant in the world.
Here’s how specific delta spike protein mutations may aid in a cell take-over:
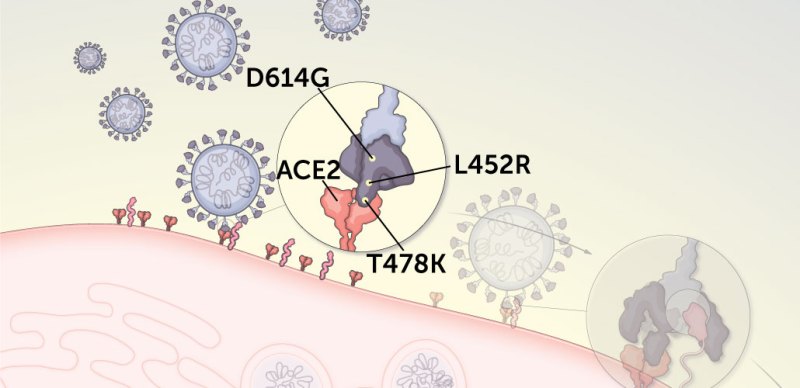
1. Some mutations allow the spike protein to get a better grip on cells.
The coronavirus begins its cellular break-in by latching onto a protein called ACE2 that studs the surface of many types of human cells. Three mutations may make delta grabbier than other variants.
D614G interrupts some molecular interactions in the spike protein near a hinge that controls whether the receptor binding domain is in a closed position where it is protected from antibodies, or in an open position so that it can grab ACE2. With the D614G mutation, it’s more likely that one or more of ACE2-snagging portions of the protein will be open for action.
L452R may strengthen the interaction between ACE2 and the spike protein, making the virus more likely to infect cells. The change switches the charge on a protein building block in a key part of the spike protein from neutral to positive. So, like a magnet attracted to metal, the mutation seems to make the spike protein bind more tightly to a part of ACE2 that has a negative charge.
T478K is unique to the delta variant. It’s so far unclear what it does, but like L452R, it may also help strengthen the spike protein’s hold on ACE2.
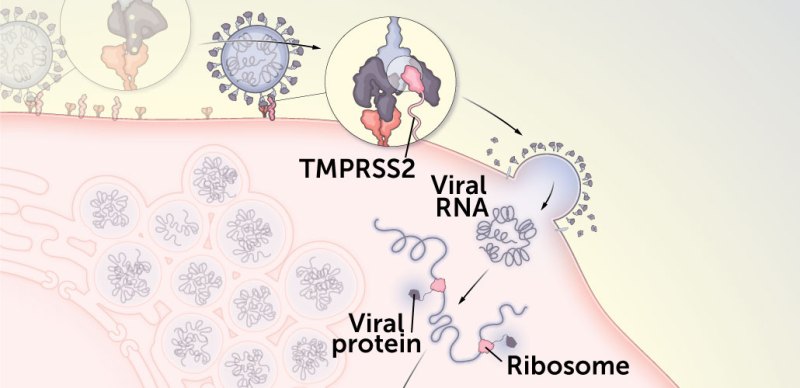
2. Some mutations allow delta to better fuse with the cell’s membrane, paving the way for the coronavirus to dump its genetic material into the cell.
Once delta latches onto ACE2, a human protein called TMPRSS2 cuts away a part of the spike protein. As a result, the S1 portion of the protein is discarded, freeing up the S2 portion of the protein to twist into shape for the next step in the process: fusing to the cell. Some evidence suggests delta may be better at letting go of S1, making breaking into cells easier.
L452R may help the virus fuse with the membrane on the outside of the cell it’s trying to infect. That allows the virus to release its genetic material and begin hijacking the cell’s machinery to begin making more copies of itself.
P681R may create a stretch of basic amino acids that could help the virus fuse better with the cell membrane, helping more viruses get inside more cells.
As a result of these and other changes, delta can fuse with cells faster and can enter cells that have lower levels of ACE2 studding their surfaces than other variants can, researchers reported in Science in October.
After the virus fuses to the cell, it sets loose its RNA and turns its new lair into a virus factory: Viral RNA is copied, human ribosomes make viral proteins and the cell churns out nearly identical copies of the coronavirus. As the virus makes copies of its RNA, it can make typos. Sometimes, the genetic errors help the virus, which can give rise to variants like delta. But not all changes are good for the virus. Some genetic typos cause damage to viral proteins, meaning the viruses with those mutations can’t infect new cells. Other changes don’t have any effect on the virus at all.
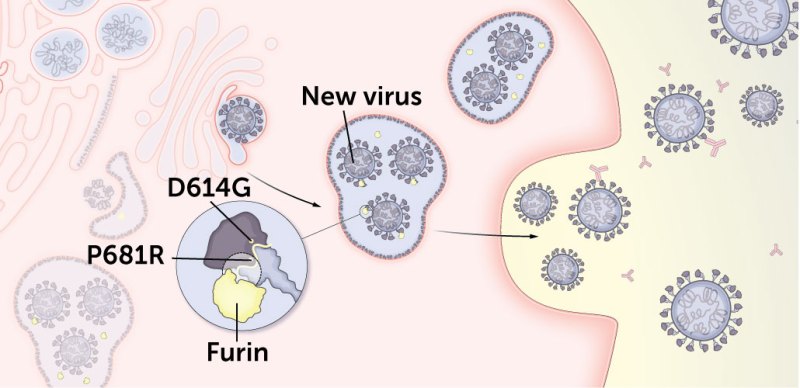
3. Some delta mutations may better prime newly made viruses to more easily infect cells.
Before newly made viruses are released from the cell, the human protein furin snips the spike protein between S1 and S2. That lets the spike protein adopt the right shape to snag human cells and sets the protein up to allow for better membrane fusion.
Delta may be snippier than other variants.
Mutations D614G and P681R may increase the number of spike proteins cut by furin on each newly made virus, better prepping the viruses to enter other cells.
D614G makes it easier for furin to make its snips. This preliminary cut happens in a different location than the TMPRSS2 cut. The mutation may also increase the number of spike proteins on each copy of the virus.
Like D614G, P681R may also boost the number of spike proteins cut by the human cell protein furin, priming the newly made viruses to infect new cells.
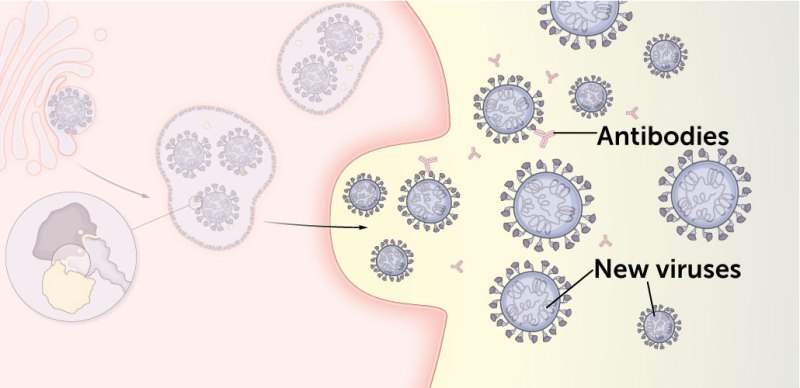
4. Some mutations may help newly released viruses evade antibodies as the viruses seek out other cells to infect.
The immune systems of people who have recovered from an infection or those who got vaccinated make antibodies to the coronavirus. Several of delta’s mutations may help the virus evade these antibodies, which would otherwise block viral entry into other cells.
T19R, G142D, R158G and two spots — called E156del and F157del — where amino acids are missing from the protein may hide parts of the virus from antibodies, helping it slip past those immune system defenses.
T478K, the mutation unique to the delta variant, is close to the same spot as E484K, a mutation that was implicated in the antibody-evasion tactics of the beta variant.