Cloning produces human embryonic stem cells
Fine-tuning of technique used in other animals could enable personalized medicine

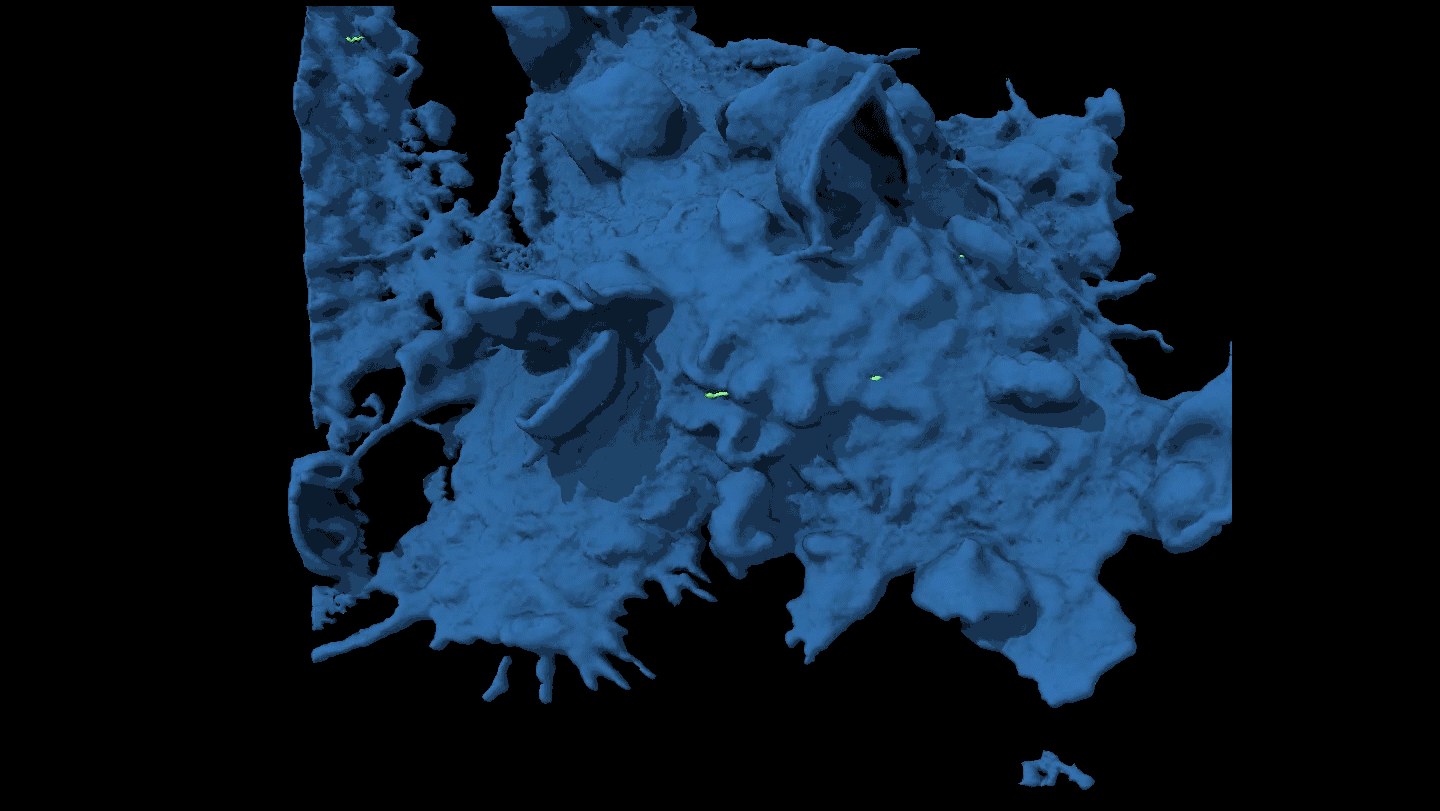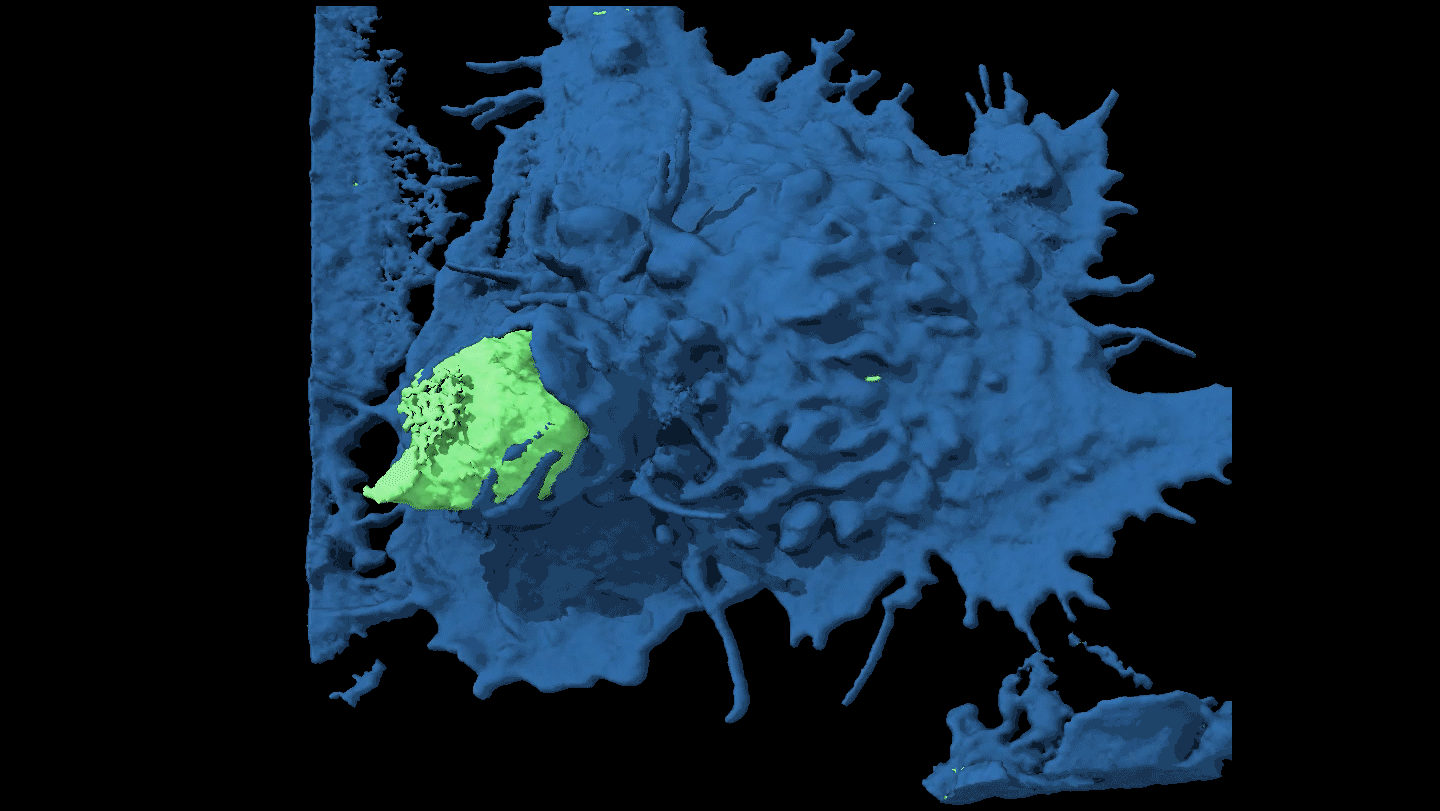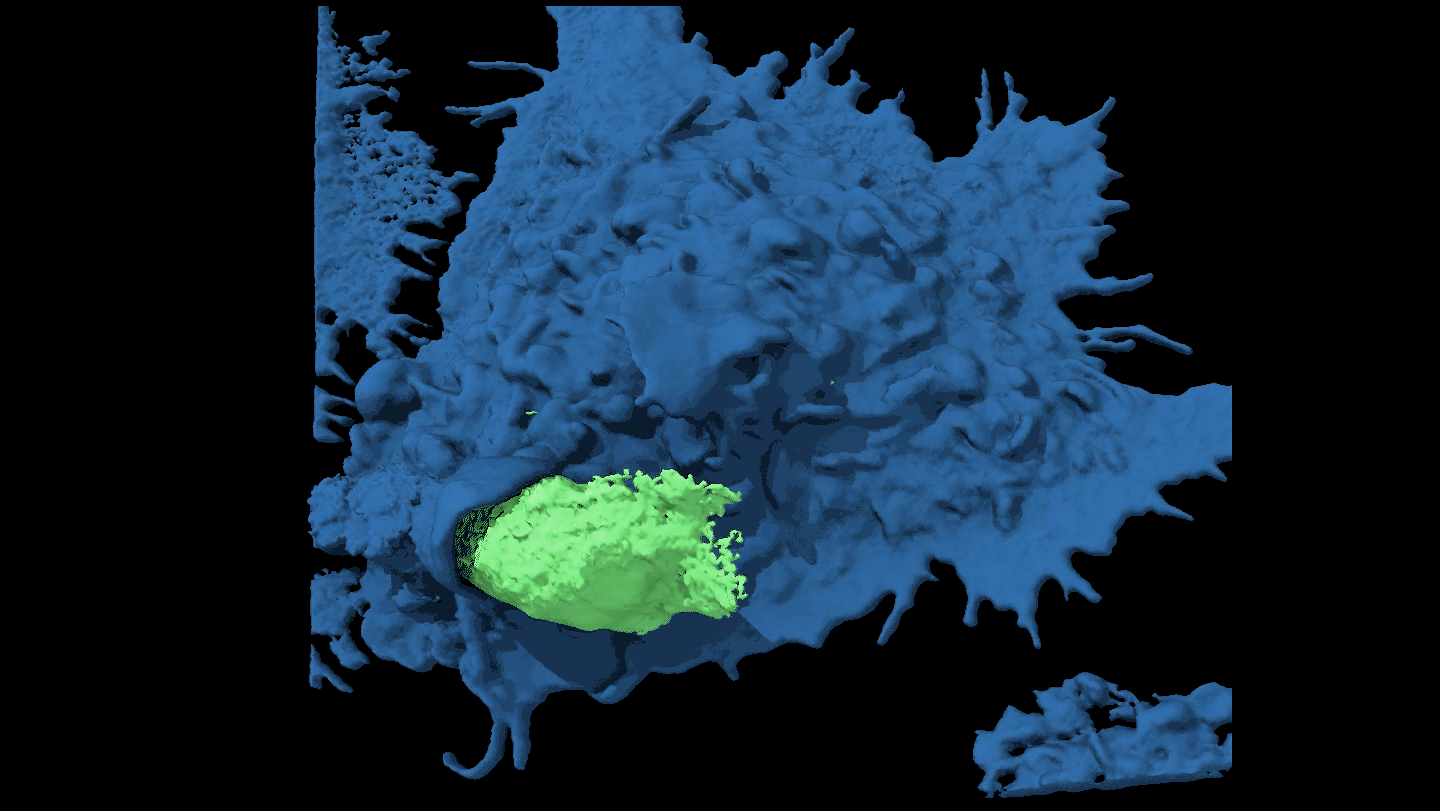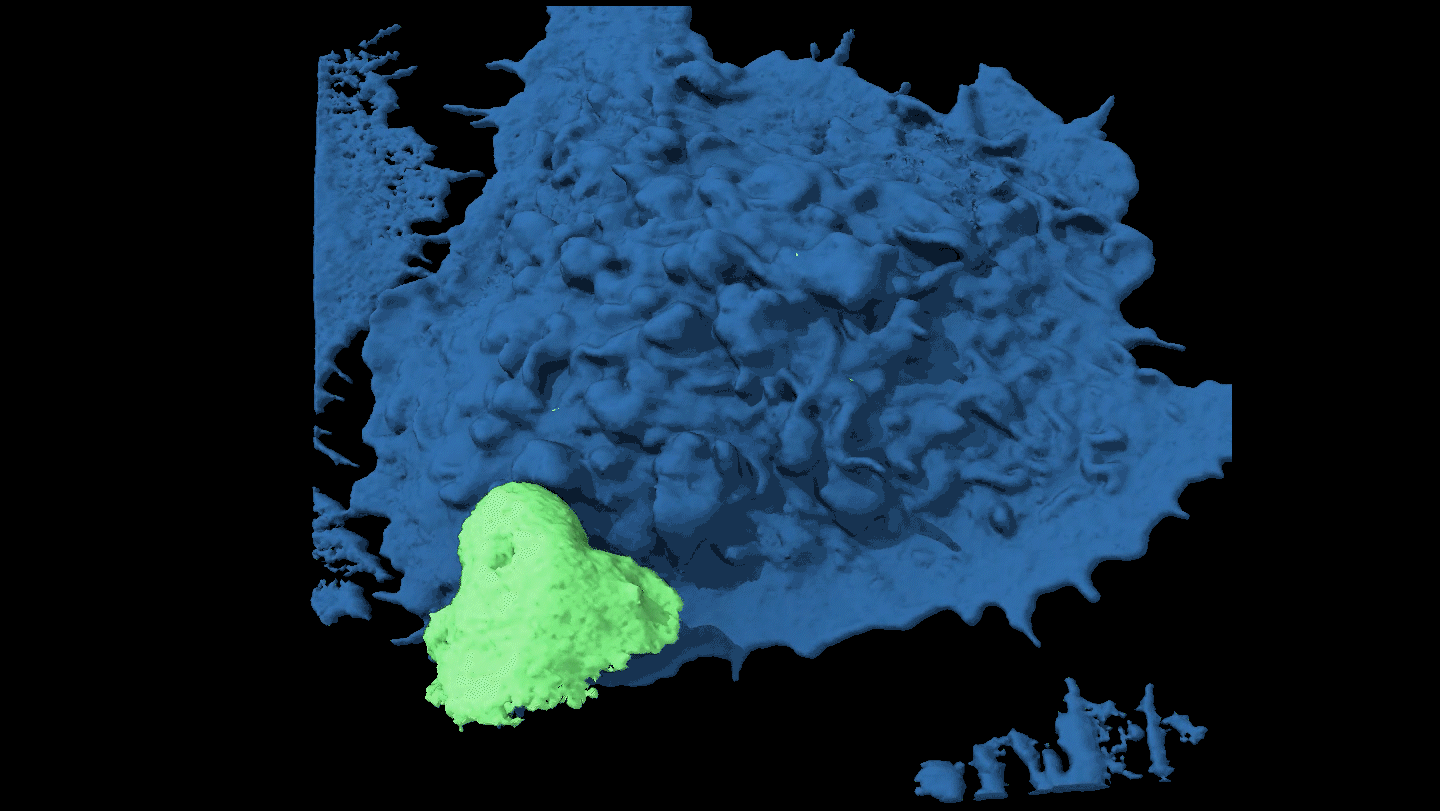
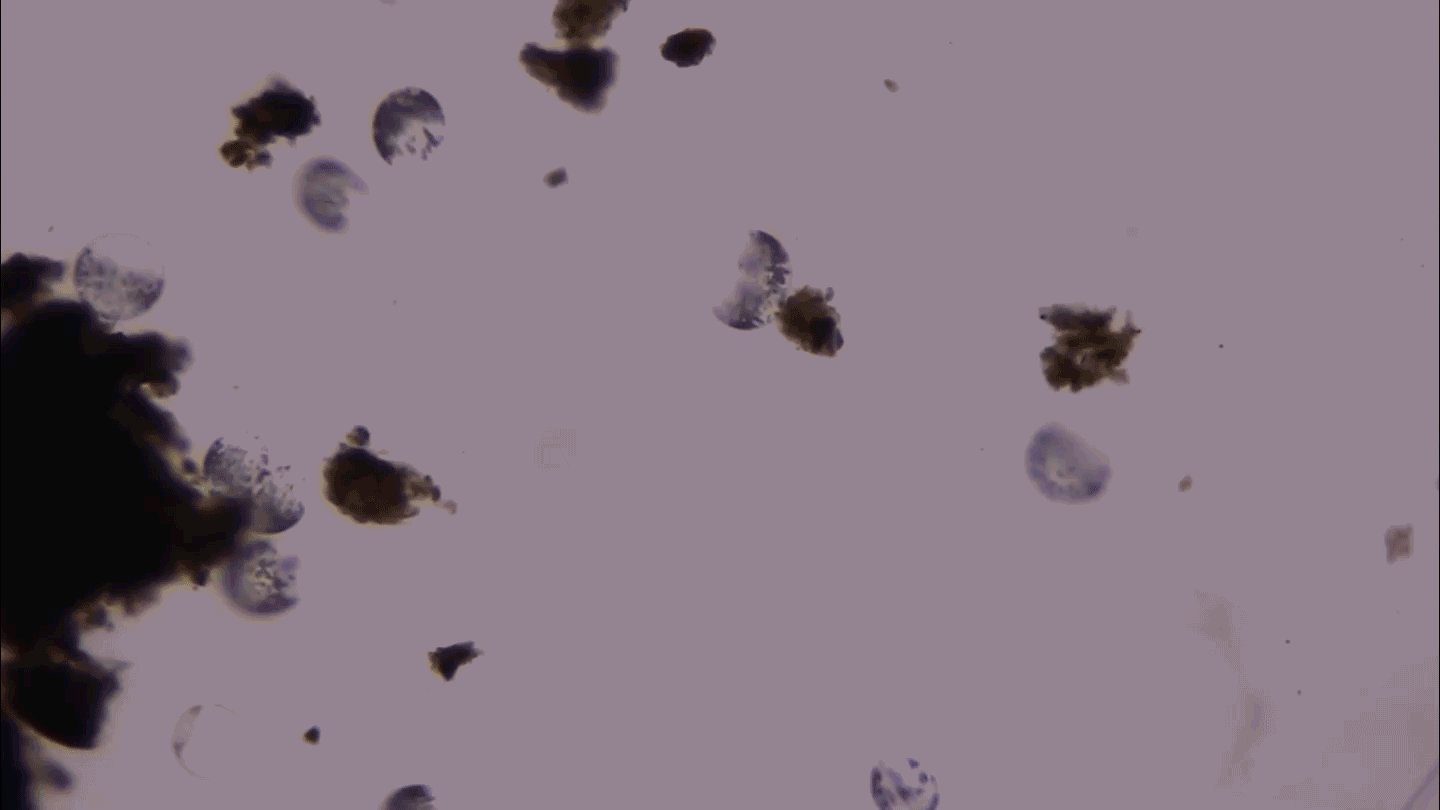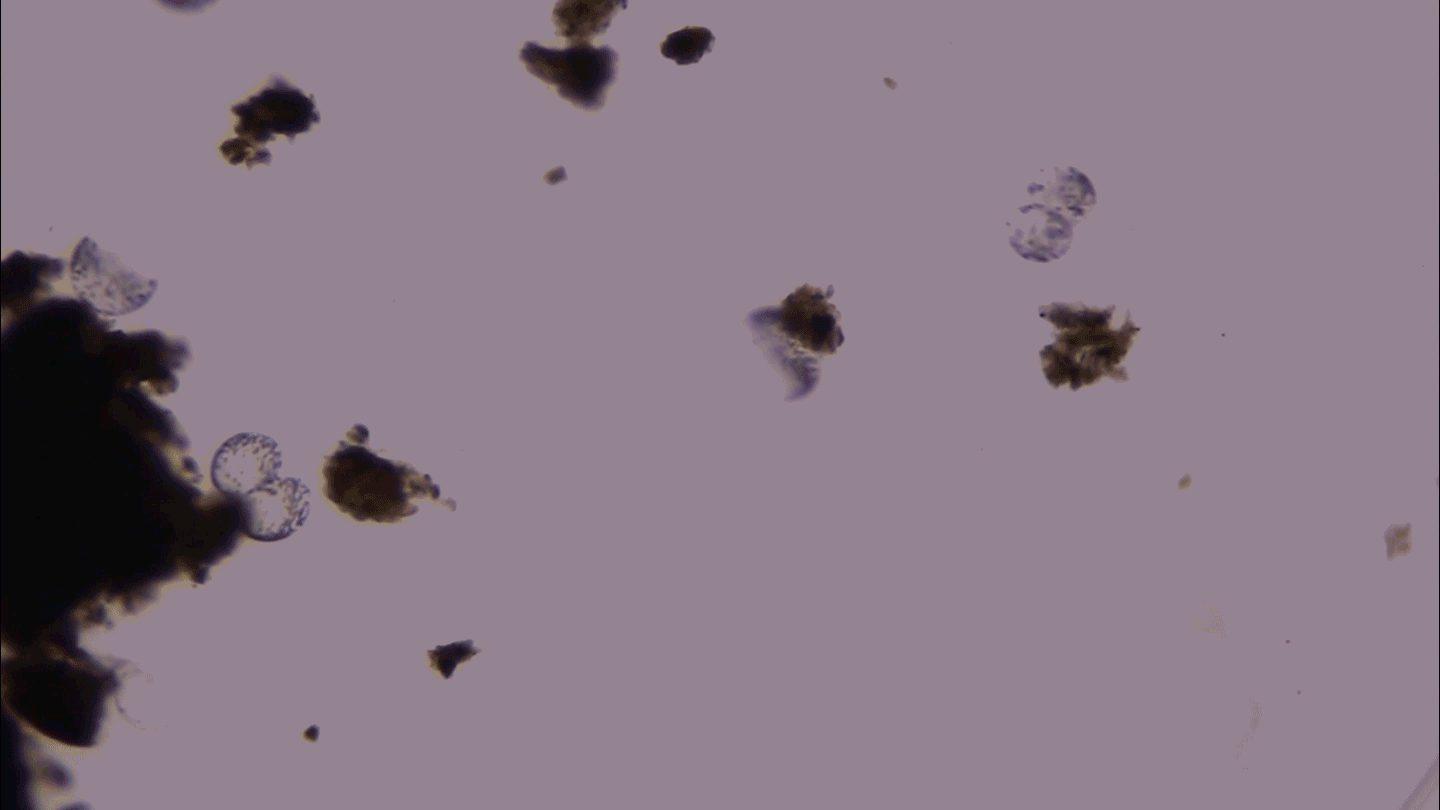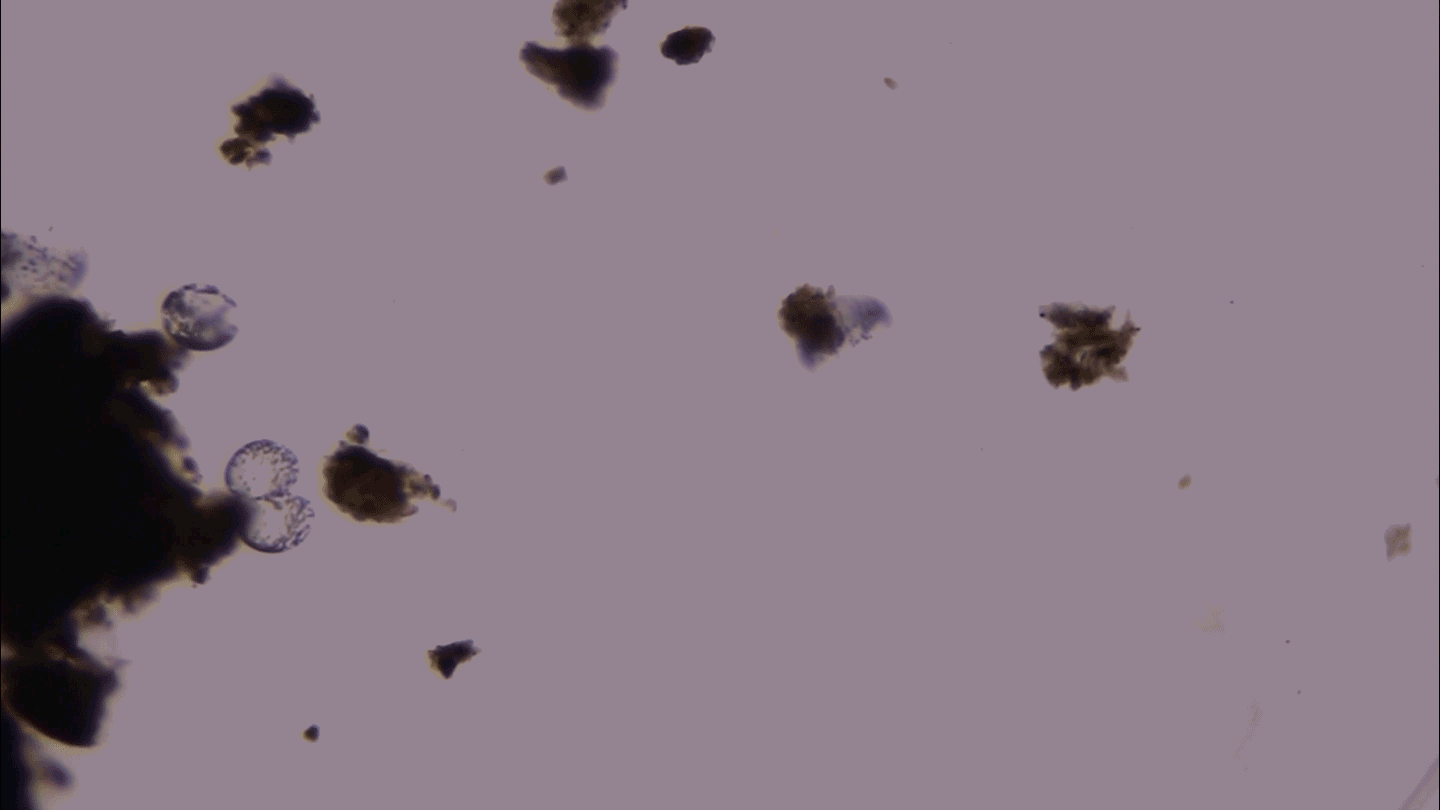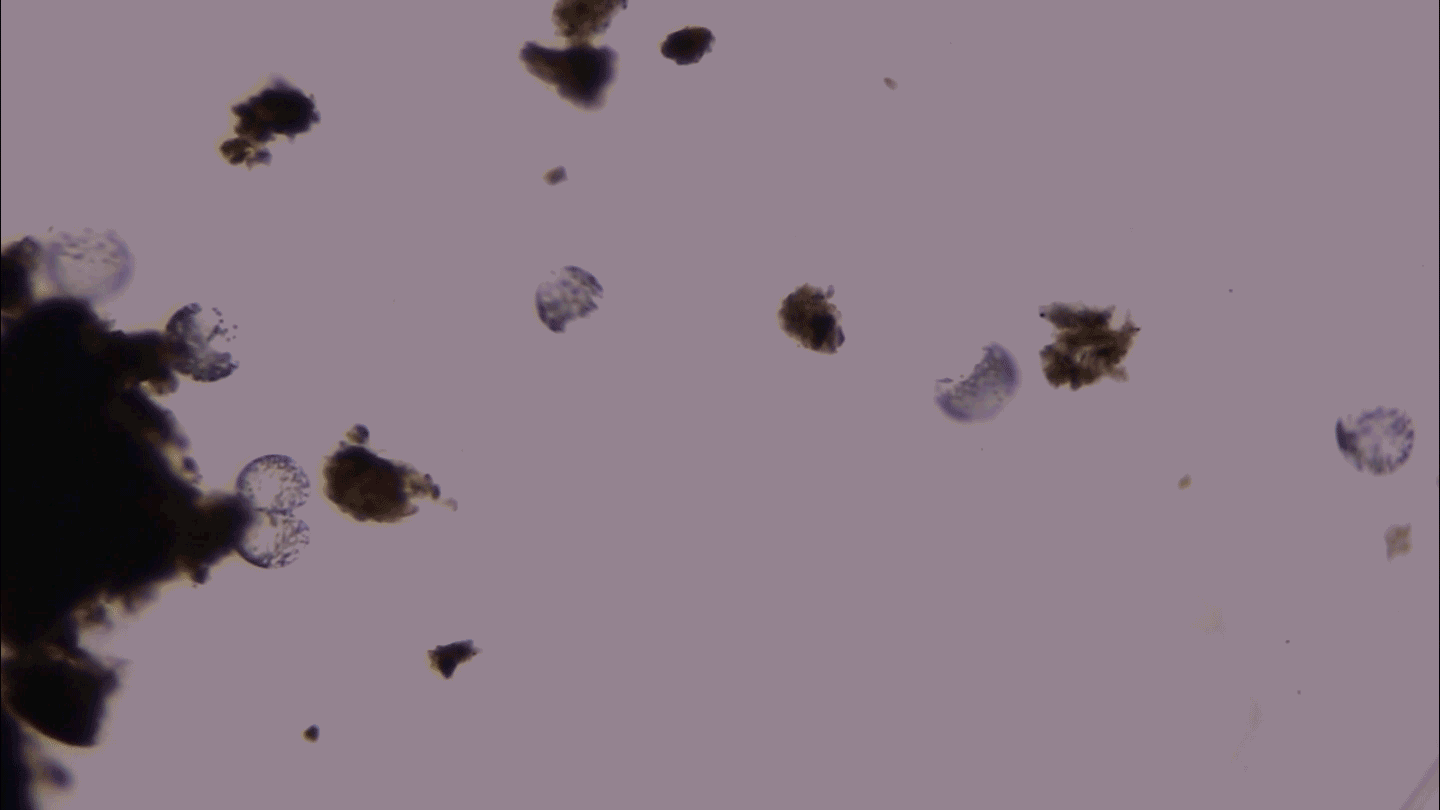
CLONING FEAT Using a laser and a tiny needle, researchers suck DNA from a human egg, the first step of a newly revised process that created human embryonic stem cells for the first time.
Courtesy of M. Tachibana
View the video
For the first time, scientists have created human embryonic stem cells by transferring the nucleus of a mature cell into an egg. The cloning technique could nudge the dream of personalized medicine closer to reality, researchers suggest May 15 in Cell.
“It’s a huge, landmark achievement,” says stem cell biologist George Daley at Children’s Hospital Boston and Harvard University. Creating embryonic stem cells by nuclear transfer in humans, he says, is “the next major technological advance since Dolly.”
The famous sheep Dolly was the first mammal cloned by the nuclear transfer technique, injecting the nucleus of a cell from one adult sheep into the egg of another. Since the animal’s birth in 1996, scientists around the world have tried to duplicate the technique in human cells.
Unlike adult cells, which have already followed a path to become, say, heart cells, neurons or skin cells, embryonic stem cells are uniquely poised to become any cell in the body. And if scientists could make these stem cells from a patient’s own tissues, once-untreatable conditions could perhaps be cured by replacing damaged cells with healthy ones.
But creating embryonic stem cells in humans has proven tricky, says Kathrin Plath, a stem cell biologist at the University of California, Los Angeles. No one knew why the technique worked in other mammals but not humans. Researchers had to figure out the best way to ease out an egg’s DNA, slip in a new nucleus and then cue the egg to divide and grow. In 2011, scientists came close, but the egg stalled out after three divisions, producing just eight cells.
In 2007, a new way to make stem cells dazzled scientists in the field (SN: 11/24/07, p. 323). By dosing human cells with a small cocktail of molecules, researchers pushed a reset button that turned adult cells back into embryonic-like ones called induced pluripotent stem cells, or iPS cells.
“For the last six or seven years, virtually all of us have ended our nuclear transfer efforts and switched over to iPS cells,” Daley says.
But a team led by Shoukhrat Mitalipov of the Oregon National Primate Research Center in Beaverton kept plugging away at nuclear transfer, first using rhesus macaques, and then with human cells.
One key change was adding caffeine to the eggs before DNA transfer, says stem cell biologist James Byrne of UCLA, who was not involved in the new work. Caffeine acts like a set of chemical reins, holding back the egg’s development until researchers inject a new nucleus. The new protocol also features other tweaks such as examining the eggs under polarized instead of ultraviolet light, which can damage the egg.
Using the new method, researchers made embryonic stem cells from an egg and the nucleus of a young boy’s skin cell. The new cells can grow and divide to form a mass of embryonic stem cells just like those derived from fertilized embryos, Mitalipov said in a press briefing May 14.
And when researchers ground the cells up and compared the genetic bits to those in embryonic stem cells, they didn’t see much of a difference. Virtually all of the new cells’ genes were reset to their embryonic states.
What’s more, Byrne says, the approach boasts “dramatically improved efficiency.” Instead of burning through thousands of eggs to make a single embryonic stem cell line, Mitalipov’s group could use just two eggs.
The new cells may have advantages over iPS cells in treating some genetic flaws that lurk in mitochondria, little cellular power plants that carry their own DNA. By putting the nucleus of a patient’s skin cell into a fresh egg with healthy mitochondria, scientists could potentially make a customized therapy that erases the defects, Mitalipov said.
The work “is certainly impressive,” says developmental biologist John Gurdon, who shared the 2012 Nobel Prize in physiology or medicine for pioneering the nuclear transfer technique to clone a frog.
Next, Gurdon says, researchers ought to compare the new embryonic stem cells with iPS cells. A side-by-side look might provide clues to how resetting adult cells actually works. If they can figure out why Mitalipov’s nuclear transfer method is so successful, researchers might be able to improve the technique to make iPS cells and avoid having to retrieve eggs from volunteers.
Embryonic stem cells made using this method have the potential to treat spinal cord injuries and diseases such as diabetes or Parkinson’s, says Dietrich Egli, a stem cell biologist at the New York Stem Cell Foundation. “I’m very confident that such cells will be used for therapies in humans in the future.”
Researchers used a modified technique to create human embryonic stem cells using adult cell nuclei and egg cells. The scientists were then able to turn the immature cells into a variety of tissue types, like these contracting heart cells.
Courtesy of Cell, Tachibana et al.
Back Story | HISTORY OF CLONING RESEARCH
Advancement in animal cloning has occurred against a backdrop of ethical debates and scientific fraud.