Daisy Martin didn’t seem sick. Come dinnertime, she was as ravenous as ever. And at the sight of a new toy, she danced around in excited circles, same as always. Then one day, when Daisy was 8 years old, one of her family members noticed a lump, and then others, on the side of Daisy’s neck, beneath her fur. The diagnosis was devastating: T-cell lymphoma, a cancer so merciless that Daisy’s family feared losing her within weeks. The one hope was to enroll Daisy in a study of an experimental drug, available more than an hour’s drive away at the University of Minnesota in St. Paul. Her family was torn. Would the treatment cause more suffering? Would she lose her hair?



Daisy (pictured below) is a doe-eyed Shih Tzu. As with research volunteers of the human kind, her participation in the drug trial meant she could obtain care that her family could not otherwise afford. She would help scientists collect data that could benefit future generations of ailing dogs, even if she herself could not conquer the cancer.
And the trial reaches beyond canine patients. Whether Daisy recovers, and how she recovers, will provide information about a drug that could one day help dog owners as much as dogs themselves.
When it comes to cancer, dogs now exist at the nexus of human and veterinary medicine. “I just came back from a meeting of the connective tissue oncology society, where two of the talks were results from studies of pet dogs. Neither of the two people presenting the talks were veterinarians,” says Chand Khanna, a veterinarian by training who heads the comparative oncology program at the National Cancer Institute, part of the National Institutes of Health in Bethesda, Md.
And last year, Harvard Medical School invited Elaine Ostrander, an NIH cancer geneticist who has long worked with dogs, to deliver the esteemed Franklin H. Epstein Memorial Lecture. Her talk, given to the faculty of Harvard’s Beth Israel Deaconess Medical Center, covered the human implications of “good dogs with bad genes.” She says that when she learned of her selection for the honor, she thought, “Hot diggity dog! We’ve made it!”
Khanna, Ostrander and other researchers with similar interests have long contended that dogs offer an under-recognized bounty for oncology. No matter the species, cancer arises when some tragic combination of a body’s genetics and its surroundings conspire to take the brakes off cell growth. Dogs’ environmental exposures occur alongside those of people: For centuries, pooches have lived with people, sleeping in their homes and eating their foods. And dogs’ genetics are not so different either. When the partial genetic instruction book for a poodle was published in 2003, followed by a more detailed look at a boxer in 2005, scientists learned that most genes in man are shared with man’s best friend. The DNA within many tumors may carry an even more similar pedigree.
“The genome data were transformational,” says Jaime Modiano, an immunologist and veterinarian at the University of Minnesota. “It allowed us to say that a cancer in dogs and a cancer in humans are the same thing.”
Canine genetic studies since have yielded important clues to how cancers of the blood, bone and brain arise. Better understanding of these diseases may lead to better treatment. The most common type of non-Hodgkin’s lymphoma, for example, affects dogs and humans in almost equal proportion, and the same chemotherapy treats both. Veterinarians have long turned to human drugs to treat their canine patients, but one day the pipeline may flow in the other direction. More and more pets like Daisy are participating in tests of new treatments, studies that produce answers sooner than human trials because dogs are shorter lived.
“There’s a lot bubbling right now,” says Kerstin Lindblad-Toh of the Broad Institute of MIT and Harvard, a leader in the project to sequence the boxer genome. “This year and the next you’re going to see a lot of publications.”
Genes that sit, stay
Animal research has long been a part of medical science. But unlike mice and rats, the dogs involved in oncology research don’t live in laboratories. They are patients, occupying a place as companions and family members. Dogs were the first animals to be domesticated, and although both dogs and humans have benefitted from the storied relationship, dogs have paid a price for their heritage.
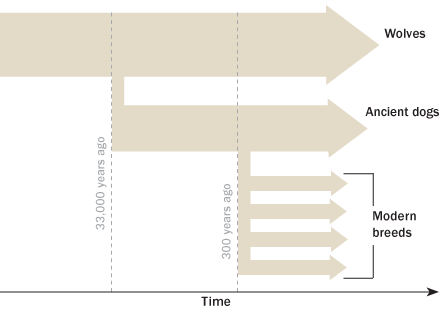
Dogs evolved from wolves, entering human culture around 33,000 years ago. Solid records date back around 15,000 years, says Matthew Breen, a geneticist at North Carolina State University. “Fifteen thousand years ago, dogs didn’t sit at our feet and watch TV. They helped the nomadic tribes survive.”
Scientists can only hypothesize about the path from feral to Fido, but Breen says one theory is that early humans began tossing food to wolves as a way of bribing against attack. Over time, wolves and people became dependent on one another; wolves grew accustomed to the easy meals, people enjoyed the protection wolves offered. As time passed, relationships deepened.
“They helped us by hunting, herding and guarding. They became companions,” Breen says. By the late Pleistocene, which ended about 11,700 years ago, humans were buried with their dogs. The ancient Egyptians mummified some dogs like royalty.
Following the transformation from wolves, the genetic milestone in dogdom came with the industrial revolution, Breen says. “The landowners and factory owners had something they never had — free time. As a consequence, we started to breed [dogs] because we liked the way they looked.” Owners developed a fancy for long or short coats, small or large bodies, or a dog’s ability to hunt or guard or just lounge around being sweet. Most breeds recognized by the American Kennel Club have been distinct genetic entities for about 200 to 300 years, Ostrander says.
All this selective breeding greatly decreased genetic diversity. Further bottlenecks came about during the lean times of the Depression and world wars, when purebred dog populations shrank. Today, many breeds such as Leonberger dogs and Portuguese water dogs are the descendants of perhaps fewer than 20 or 30 founders.
Along the way, as people handpicked the most attractive dogs for breeding — often mating uncles and aunts with nieces and nephews — they unknowingly amplified hidden genetic triggers for cancer.
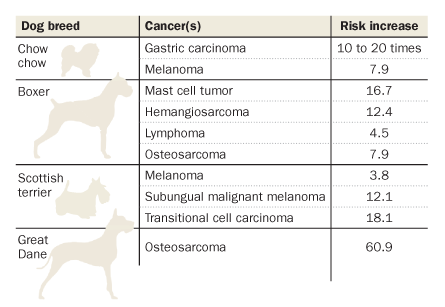
About 25 percent of dogs will eventually develop a malignancy, Breen says, and the proportion is increasing. Cancer kills about 13 percent of the world’s human population, and some dog breeds have a cancer-related death rate three or four times that. About half of all golden retrievers die from cancer, commonly lymphoma. Large breeds are known for their high risk of bone cancers, while brain tumors are typical among dogs with squished-looking faces, including pugs and bulldogs.
101 mutations
Yet the same genetic inbreeding that makes dogs so cancer-prone also provides an easier landscape to search for cancer’s roots. In appearance, solid tumors removed from people and dogs can be practically indistinguishable. “You couldn’t tell a difference between a dog tumor and human tumor under the microscope,” says Peter Dickinson, a veterinary neurosurgeon at the University of California, Davis. The molecular engines of disease are also often similar.
To find genetic flaws responsible for human disease, scientists have to study hundreds, even thousands of people to account for the random, irrelevant genetic background noise. Imagine hearing a symphony, straining to listen for just the viola. You’d need to listen over and over, slowly eliminating every other instrument. The task would be easier if the violas were louder and more numerous, and had fewer violins and cellos drowning them out. Centuries of inbreeding have given dogs a simpler genetic ensemble, so scientists have an easier time finding a distinct melody.
Consider that one search for a genetic cause of gliomas, a deadly brain tumor, involves more than 700 human patients and almost 4,000 healthy volunteers in a comparison group. Dickinson and his research team are performing a parallel and probably equally powerful study with just 40 affected dogs and 150 healthy ones. They have narrowed the search for their glioma gene down to one chromosome.
Other canine gene hunts are under way. In 2012, a team of researchers that included Ostrander and Breen announced the location of a gene for histiocytic sarcoma, a form of lymphoma that is rare in humans but strikes up to a quarter of Bernese mountain dogs and flat-coated retrievers. It is an aggressive malignancy with no cure. In a cover article in Cancer Epidemiology, Biomarkers and Prevention, the researchers described a genetic mutation that appears to be associated with the cancer in these dogs.
Importantly for their masters, the aberration turned up in a poorly understood genetic region that has been associated with other kinds of human malignancies — yet another clue in the search for cancer’s origins. “People are dogs, in terms of cancer,” Breen says.
A 2009 study by Khanna, from the National Cancer Institute, shows how true that may be. The analysis, published in BMC Genomics, found that the genes active in human osteosarcomas (the most common malignant bone tumor in children) and dog osteosarcomas are almost indistinguishable. If the genes are the same, the treatment can be, too. The following year, he reported in PLOS ONE that the drug rapamycin fights the cancer the same way in dogs as it does in people.
Better treatments unleashed
That finding is good news for dogs, but there’s a larger point. Dogs have long relied on human chemotherapy drugs, but as studies find more and more instances of tumors that are almost genetically interchangeable, medical researchers are turning to dogs to test drugs for humans.
Mice and rats have long stood in for people, but four decades of the war on cancer have revealed major shortcomings to this approach. Rodents do not develop human cancers naturally, so researchers often have to graft tumors from people into mice and rig unnatural arrangements such as severe immune suppression that allow the cancers to grow.
Still, the tumor isn’t inclined to this kind of existence. That’s why cancer is cured many times over in labs, but not so often in actual people. The late Judah Folkman, a renowned researcher who pioneered a new class of oncology drugs, once said, “If you have cancer and you are a mouse, we can take good care of you.”
By contrast, dogs have cancers that arise spontaneously in the face of an intact immune system and a normal life. While no one is considering eliminating rodent research, people are thinking about turning more to dogs, especially for modern treatments that try to engage the body’s own immune system in the fight.
In 2010, the U.S. Food and Drug Administration approved one of the first medicines to use this immune system–enlisting approach, the prostate cancer drug Provenge. The treatment amplifies an immune attack by exploiting the body’s own antigen-presenting cells. These cells consume proteins that don’t belong in the body, grind them up and then display them on their surface to alert the immune system.
“The immune system is rather polite,” says Nicola Mason, an immunologist in the veterinary school at the University of Pennsylvania in Philadelphia. “It won’t attack anything until it’s been properly introduced.” For Provenge, doctors harvest a type of antigen-presenting cell called a dendritic cell from a patient, incubate those cells with the prostate cancer cells, and infuse the newly enhanced dendritic cells back into the patient.
But in many ways, dendritic cells are less than ideal for making a cancer vaccine, says Mason, who is trying to improve on the method through research in dogs. The Provenge regimen is time consuming and expensive, costing about $93,000 per patient. Mason is researching the same concept with a different kind of immune cell, called a B cell. The treatment doesn’t require so many cells, and is easier to carry out.
In 2011, in PLOS ONE, Mason published a study of 30 dogs with lymphoma, testing the effectiveness of using B cells in immune therapy. When dogs get lymphoma, they often go into remission after chemotherapy but then relapse about 90 percent of the time, she says. In the Penn study, all dogs were treated with standard chemotherapy, but 19 of them also received the immune therapy after they achieved remission. Four vaccinated canine patients have never relapsed. “This tells us that in some cases you can induce a cure,” she says.
Among the dogs receiving the immune therapy that did relapse, 10 received a second round of chemo; 40 percent of those (4 more dogs) achieved remission, compared with only about 8 percent of a comparison group of 39 unvaccinated dogs that received another round of chemo treatment after relapse.
It’s still too early to know how studies like this will advance human oncology, but dog owners are delighted when they see signs of benefits, as Ostrander says, at both ends of the leash. In December at a meeting of the American Society of Hematology, the research team presented the results of the study that included Daisy. The experiment was carried out in three veterinary schools and sponsored by the biotech company Karyopharm, based in Natick, Mass. The 14 dogs were given gradually larger doses of the experimental drug to test whether it would have any major unanticipated side effects. None appeared, and studies are continuing. Meanwhile, studies of a human counterpart are also under way.
As for Daisy, she is doing well. “We’re so thankful for every single day we have with her,” says her owner, Cindy Martin. Daisy’s part in cancer history may be minor, but at home, she will always be a star.
Cancer research goes to the dogs
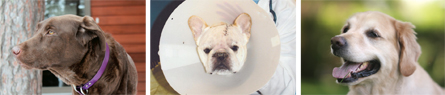
From left: Courtesy of Jan Takaichi; Courtesy of Susan Bunyard; Courtesy of Barry Doohan
People in clinical studies help the next generation of cancer patients. Canine efforts help not only future generations of dogs but people, too. Here are some four-legged patients who benefited from experimental therapies for their cancers:
Kodiak (left) was 8 years old when he developed a limp in his left front leg. An X-ray found that his wrist was riddled with cancer. His owners took him to the University of Minnesota, where his leg was amputated and he received treatment with a gene therapy drug that is also in human trials for liver cancer. Today he is back duck hunting and cancer free.
A French bulldog, Devon (middle) was diagnosed with a brain tumor when he was just 18 months old and starting a champion show career. Veterinarians gave him only two months to survive with the aggressive malignancy. He received an experimental chemotherapy injected into the tumor at the University of California, Davis and lived another 21 months.
He died August 12, 2010.
Barney’s (right) lymphoma was already advanced when his owners enrolled him in an immune therapy study at the University of Pennsylvania in Philadelphia. Though lymphoma usually relapses in dogs after treatment, Barney was one of four patients whose cancer disappeared for good. Six years after treatment, he is now 13 years old and shows no signs of cancer.







