Blind mice see again
Bacterial gene restores sight in experiments
- More than 2 years ago
Researchers have restored sight to blind laboratory mice by using gene therapy. The new treatment, published online June 24 in Science, may one day allow some people with retinitis pigmentosa, an incurable genetic eye disease, to read, drive and navigate a room.
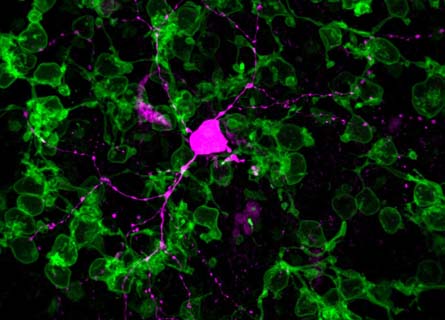
“It’s an excellent study,” says developmental neuroscientist Connie Cepko of the Harvard Medical School, who was not involved with the research. “This is conceptually a nice way to try to restore or prolong vision.”
Retinitis pigmentosa causes tunnel vision and night blindness in 2 million people worldwide. It primarily wipes out the outer retina’s rod cells, which are highly sensitive to light and allow night and peripheral vision.
Some patients also lose daylight vision and go blind completely, because the color-sensing cone cells of their inner retinas slowly degenerate. But the disease doesn’t kill cone cells immediately; it first makes them nonresponsive to light. That means there is a window of time where the cone receptors are still there, but not functioning.
So scientists at the Friedrich Miescher Institute for Biomedical Research in Basel, Switzerland, attempted to reanimate diseased cone cells in mice. Using a virus already approved for human gene therapy, the researchers inserted a gene from a light-sensitive bacterium, Natronomonas pharaonis, into cone-cell DNA.
The gene is a blueprint for proteins that form passageways in cell membranes. When stimulated by light, those proteins open up and let negatively charged ions into the cell. When inserted in the mouse cell membranes, these proteins helped mimic the normal activity of healthy cones.
Not only did the restored cone cells respond to light, but they also sent signals to the brain so the mice could see.
“What was really astounding is that these cells that were blind for a while were still connected to the rest of the circuit,” says neurobiologist Botond Roska, who led the study.
But unlike healthy cone cells, the restored cone cells could not adapt to different light levels. The cells responded best to bright yellow light similar to sunlight at the beach, Roska says. In order for human patients to see in dimmer light, researchers would have to develop special glasses with light-sensing cameras to adjust the intensity of light projected to the patients’ eyes, he says.
After more studies with mice and primates to ensure its safety and effectiveness, Roska aims to make the treatment available to human patients.
“This is not a treatment for all patients with retinitis pigmentosa,” Roska says, “but for a subgroup in which the cone receptors are still there.”
His team will also explore how long the therapeutic effects last and whether the gene therapy could have applications for other eye diseases like macular degeneration and retinal damage due to diabetes.
“I have a feeling it will make it into human trials,” says Cepko. “How a person will perceive these signals is hard to say, but it should do at least as well as it did for mice.”