By exploiting a defect in a semiconductor’s crystal structure, researchers have come up with a potentially inexpensive way to make fast fiberoptic communications components. That development, in turn, might speed the long-awaited extension of optical networks into homes, says Janet L. Pan of Yale University.
Working with gallium arsenide, the primary material from which lasers for compact-disk players and the high-speed electronics for cell phones are made, Pan and her colleagues have created a light-emitting diode (LED). The device converts electric pulses into light emissions at the pivotal infrared wavelength of 1.55 micrometers (m), the one used for long-distance optical-fiber communications. Ordinarily, gallium arsenide emits at 0.85 m.
Many manufacturers currently use indium phosphide for making LEDs, lasers, photodetectors, and other components of fiberoptic systems. However, for electronics, indium phosphide is difficult to use and leads to many defective components that can’t be sold.
By contrast, because gallium arsenide parts can be fabricated in the same automated processes that create gallium arsenide–based microelectronics, the new fiberoptic devices may prove to be relatively cheap, Pan says.
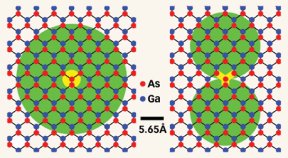
In the June Nature Materials, the Yale team explains how it exploited gallium arsenide defects known as arsenic anti-sites. At those locations, the scientists introduced extra arsenic atoms to replace some gallium atoms in the compound’s crystal lattice. In that manner, the team built an LED that contains a layer especially rich in arsenic anti-sites.
In LEDs, mobile electrons drop from higher to lower energy levels and fire off photons whose wavelengths correspond to the differences between those levels. In gallium arsenide, anti-sites permit electrons to assume intermediate energy levels that aren’t otherwise present. When electrons drop into those middle levels, they emit photons with less energy, and therefore longer wavelengths, than usual.
The new LED is “a clever way to make use of a very high concentration of defects,” comments David C. Look of Wright State University in Dayton, Ohio.
For the moment, Pan notes, the LED’s light-producing efficiency is too low to be useful, adding that “practical devices may be possible within 3 to 5 years.”
****************
If you have a comment on this article that you would like considered for publication in Science News, send it to editors@sciencenews.org. Please include your name and location.