- More than 2 years ago
Lead poisoning has serious health consequences in adults, including brain and kidney damage, and causes various developmental problems in children. That’s why lead testing has become an important public health measure.
Commercially available tests for detecting lead in household paint sometimes give incorrect results, says Yi Lu of the University of Illinois at Urbana-Champaign. More sophisticated tests for the toxic metal can be more reliable, but they require expensive equipment and expertise.
In the June 4 Journal of the American Chemical Society, Lu and Juewen Liu, also of the University of Illinois, describe a reliable sensor that uses a simple color change to indicate the amount of lead in paint. The scientists devised the sensors from gold nanoparticles and tailor-made DNA strands.
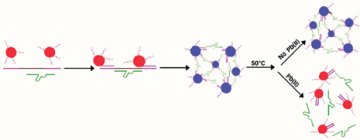
GOLDEN HUES. In leadfree environments, gold nanoparticles (red) adorned with short DNA strands assemble onto longer strands of DNA (purple) and aggregate into a structure that appears blue. In the presence of lead (Pb) ions, catalytic DNA (green) snips the longer DNA strands. This prevents aggregation, and the gold particles appear red. |
To conduct a test, chips of either water- or oil-based paints are dissolved in dilute vinegar and a drop of the liquid is added to a test tube containing the sensors. A drop of this solution is then placed on an alumina test plate. If the solution contains lead, it produces a purplish or red spot. Lead-free drops produce blue spots.
To make their sensors, the researchers use three ingredients: 13-nanometer-wide gold particles adorned with short strands of DNA; strands of so-called catalytic DNA, which can snip apart other DNA, strands; and longer DNA strands to which the first two ingredients attach.
In the absence of lead, the catalytic DNA doesn’t break up the longer DNA, and the bound nanoparticles aggregate into a structure that appears blue. However, in the presence of lead, the catalytic DNA snips the longer DNA, separating the nanoparticles. Then, the particles don’t aggregate, and they appear red. With low concentrations of lead, some gold particles aggregate and others don’t, resulting in intermediate purple colors.
High concentrations of lead in paint can overwhelm sensitive detectors. For that reason, Lu makes sensors for a range of lead concentrations by replacing some of the catalytic DNA with similar strands that don’t snip DNA. These sensors require more lead ions to trigger a red response.
“It’s really innovative and exciting research,” says James Storhoff of the Northbrook, Ill., company called Nanosphere, which was founded by Northwestern University researchers Robert Letsinger and Chad Mirkin. Several years ago, Storhoff, Letsinger, Mirkin, and their colleagues invented a similar system for detecting DNA. According to Storhoff, Lu’s new sensor is remarkably selective for lead ions.
Homeowners might use the alumina plates or paper strips and a solution of the nanoparticle-based sensors to test their walls for lead, says Lu. Eventually, by using other catalytic DNA strands, these household kits may test for a variety of metal ions, including mercury, arsenic, and chromium, he says.
****************
If you have a comment on this article that you would like considered for publication in Science News, send it to editors@sciencenews.org. Please include your name and location.