Bright Lights, Big Cancer
Melatonin-depleted blood spurs tumor growth
In late 1987, Richard G. Stevens, then at Pacific Northwest Laboratories in Richland, Wash., typed up a short letter and mailed it to Walter Willett at Harvard Medical School in Boston. The two epidemiologists had met just once, and Stevens wasn’t confident that his 209-word note, or the suggestion that it contained about a possible contributor to breast cancer, would inspire any action.
But Willett took the suggestion seriously. He and his colleagues began a study that only they could do. They run the Nurses’ Health Study, a project unrivaled in scope and duration that tracks how women’s health relates to diet, activity, and other factors.
Several years later, members of Willett’s team reported that women who frequently work night shifts seem predisposed to develop breast cancer.
It was just as Stevens had suspected. He had hypothesized that nighttime illumination, by interrupting the body’s mainly nocturnal production of the hormone melatonin, might increase the risk of breast cancer. Animal experiments and surveys of people over the past 2 decades supported that hypothesis without proving it, says Stevens, currently at the University of Connecticut Health Center in Farmington.
“Now, a watershed study has provided the first strong experimental support,” Stevens says.
A woman’s blood provides better sustenance for breast cancer just after she’s been exposed to bright light than when she’s been in steady darkness, researchers led by David E. Blask of the Bassett Research Institute in Cooperstown, N.Y., report.
“Light at night is now clearly a risk factor for breast cancer,” Blask says. “Breast tumors are awake during the day, and melatonin puts them to sleep at night.” Add artificial light to the night environment, and “cancer cells become insomniacs,” he says.
“Sleep per se is not important for melatonin,” says Russel J. Reiter, a neuroendocrinologist at the University of Texas Health Science Center in San Antonio. “But darkness is.”
The new study has far-reaching implications, says Reiter. First, it could spawn trials that test whether malignancies can be slowed down by altering a person’s light environment or by using melatonin supplements. Second, he says, similar studies could show whether exposure to nocturnal light poses a prostate cancer risk to men, as some researchers suspect, or promotes other cancers previously linked to light at night (SN: 8/28/04, p. 141: Available to subscribers at Bright nights kindle cancers in mice).
When cancer awakens
Melatonin forms in the pineal gland, located in the brain, and circulates in the bloodstream. Blood concentrations of the hormone rise after dark from low daytime values and usually peak in the middle of the night.
Because the pineal gland responds to signals transmitted by the optic nerves, bombarding a person’s eyes with bright light during the night can erase the usual nocturnal surge and lower the overall melatonin production for the day. That observation concerned researchers, in part because melatonin has slowed breast cancer growth in lab experiments.
Then, there’s the disturbing circumstantial evidence.
“Breast cancer is epidemic in the world. It’s increasing everywhere,” says Stevens. It’s most prevalent in industrialized countries, where electric lights are widely used, he says. “It’s increasing very rapidly in places that are industrializing,” he adds.
Furthermore, compared with other workingwomen, female night-shift workers have about a 50 percent greater risk of developing breast cancer, says William Hrushesky of Dorn Veterans Affairs Medical Center in Columbia, S.C.
Blind women, by contrast, have unusually low rates of breast cancer and high average melatonin concentrations, he says.
“Almost nobody who does shift work adapts to it,” Stevens says. On their days off, most shift workers concentrate their activities during daylight, which upsets their circadian rhythms as much as commuting across several time zones would, he says.
That presumably explains why the original Harvard study of nurses, which was led by Willett’s colleague Eva S. Schernhammer, found that shift workers had an elevated risk of breast cancer (SN: 11/17/01, p. 317: Available to subscribers at Cancer risk linked to night shifts).
More recently, Schernhammer and her Harvard colleague Susan E. Hankinson found that women who happen to have above-average melatonin concentrations are relatively unlikely to develop breast cancer.
The Harvard researchers estimated nurses’ peak nightly melatonin concentrations by measuring the hormone in the first urine void of a day. “Those with higher levels seem to have lower breast cancer risk,” Schernhammer says. She and Hankinson reported the data in the July 20, 2005 Journal of the National Cancer Institute.
An earlier study didn’t find the same statistical relationship, but it had involved melatonin measurements in urine samples taken later in the day. Such samples are less likely to correlate with nocturnal hormone concentrations, says Schernhammer.
She notes that light is not the only relevant factor. Age and obesity both reduce a person’s melatonin production, and heavy smoking may do the same, she says. She and other researchers will report the first data that support the smoking-melatonin relationship in the March Journal of Pineal Research.
Breast cancer is less common in women who sleep more than 9 hours per night than in women who sleep less, Stevens and six colleagues in Finland report in the Oct. 15, 2005 Cancer Research. They compared cancer incidence in 12,222 Finnish women whose average nightly sleep duration had been recorded in 1975 and 1981. By 1996, 242 of the women had developed breast tumors.
Women who consistently slept 9 or more hours per night had less than one-third the risk of developing a breast tumor than women who slept 7 or 8 hours per night.
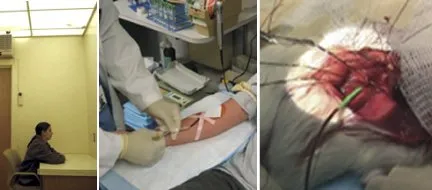
Now, Blask and his collaborators at several institutions have pushed beyond studies finding correlations among cancer, light, and melatonin. At Thomas Jefferson University in Philadelphia, researchers led by George C. Brainard asked each of a dozen healthy female medical students to give three blood samples, one during the day and two at night.
The first nighttime blood draw occurred at 2 a.m., after each woman had been in complete darkness for 2 hours. Then the volunteers stared at a brightly lit, white wall for 90 minutes, and the second nighttime draw took place at 3:30 a.m.
As expected, blood from the 2 a.m. samples contained the highest concentrations of melatonin, and daytime blood contained the lowest. Brainard then sent the samples to Blask for an unusual test of their effect on human-cancer cells.
In Cooperstown, Blask and his colleagues had implanted human breast tumor into rats in such a way that a single artery fed a tumor and a single vein received all blood leaving the cancerous tissue. The team then put plastic tubes into the two vessels, creating external conduits to and from the tumor. The researchers also shut the cancerous tissue off from the rest of the rat’s circulatory system.
Next, they pumped each blood sample from the Philadelphia medical students into a separate rat’s arterial tube and collected the liquid as it came out of the cancerous human tissue. By comparing what blood components went in and what came out, the researchers assessed the tumors’ responses to the concentrations of melatonin in the samples. For example, they measured the tumors’ uptake of H3-thymidine, an ingredient of DNA that reflects cell division and replication in a tumor.
The results indicated that the tumor cells divided most rapidly when supplied by blood taken from women either in daylight or at night after exposure to the bright artificial light. Those blood samples had low melatonin concentrations. Spiking the samples with synthetic melatonin removed their capacity to promote cancer.
Moreover, melatonin-rich blood from women who had been in darkness spurred cell division only when the researchers added a chemical that blocks melatonin’s biological activity.
In further experiments, Blask’s team determined that melatonin blocks cancer cells’ metabolism of linoleic acid, a polyunsaturated fat that’s abundant in food. The same team had previously shown that 13-hydroxyoctadecadienoic acid, the product of linoleic acid metabolism, spurs cancer cells to divide.
The team reports its results in the Dec. 1, 2005 Cancer Research.
The unusual test in the rats shows “close to conclusively” that light-induced suppression of melatonin promotes breast-tumor growth, says Schernhammer.
Stevens adds that Blask’s new technique of testing people’s blood on human tumors in animals is a powerful tool for evaluating the effect of all sorts of actions. Eating a particular food or inhaling a pollutant, for example, could alter the blood concentrations of substances that promote or fight cancer.
Managing melatonin
In the United States, synthetic melatonin is sold over the counter as a dietary supplement. Blask and other researchers want to see tests to assess whether the hormone in this form can ward off breast cancer in women. But they warn that it would be premature for people to take the hormone for that purpose.
“I personally would be pretty cautious about taking over-the-counter melatonin supplements,” says Scott Davis, an epidemiologist at the University of Washington in Seattle. “Melatonin supplements are not regulated” the way drugs are, he notes. “There may be all kinds of impurities and contaminants.”
Although synthetic melatonin hasn’t been shown to be dangerous, it could have adverse effects on the production of reproductive hormones, cautions Schernhammer.
Hrushesky is currently testing the possible benefits of melatonin supplements in men who have undergone surgery for prostate cancer. For now, though, he encourages people to opt for commonsense measures to ensure they get nightly melatonin spikes. Those precautions include going to sleep in the dark at a consistent time each night, exercising regularly, and avoiding evening use of melatonin-suppressing substances, including alcohol and medications such as beta-blockers.
People’s behavior after bedtime also counts. “They should avoid even brief intervals of [bright] light at night,” says Reiter. “A nightlight is generally safe,” he adds, because dim light has relatively little effect on melatonin.
Schernhammer offers similar advice: “If [getting up] to go to the bathroom, avoid turning on the light, or keep it dim.”
But other scientists say that it’s unclear how much a quick trip to an illuminated bathroom affects melatonin in the blood. “It’s probably inconsequential,” says Mark Rea, director of the Lighting Research Center at Rensselaer Polytechnic Institute in Troy, N.Y.
Reiter offers some other strategies for maintaining melatonin production. Blue or white light suppresses melatonin more effectively than red or yellow does (SN: 4/16/05, p. 253: Available to subscribers at Blue light keeps night owls going), so lights could be designed to filter out the offending wavelengths, Reiter says. Or people could strategically don tinted, wraparound glasses to achieve the same result, he says.
Night-shift workers face fundamental challenges, Blask says. “Melatonin works, to a large degree, by inhibiting the cancer cells from taking up linoleic acid,” he says. Cravings for fatty foods frequently assail workers in the middle of the night. As a result, many shift workers consume large amounts of linoleic acid just when their melatonin production is suppressed and unable to protect them from the polyunsaturated fat, he says.
In addition to its direct effect on breast cancer, melatonin may indirectly combat tumor growth, says Davis. Melatonin suppression encourages the ovaries to produce estrogen and other female sex hormones, which support the growth of cancerous or potentially cancerous cells in a woman’s breasts.
If future studies demonstrate such indirect hormonal effects, they’ll reveal yet one more way by which nighttime light exposure feeds cancer.







