The Body Electric
A natural voltage within a growing embryo may teach it left from right
As anyone who has ever recited the Pledge of Allegiance will attest, having your heart in the right place means having it on your left side. Despite the outward symmetry of the human body, left-right differences abound beneath everyone’s skin. The majority of the heart’s bulk usually sits on the body’s left side, although the organ’s aorta loops to the right. The right lung has three lobes, while the left has two. The liver and gallbladder fill up the right side of the abdomen, whereas the spleen and stomach dominate the left.
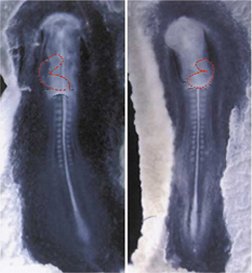
In rare cases, about 1 in 8,500 people, a person’s internal organs are completely flipped across the left-right axis–for example, the spleen is on the left, not the right.
Known as situs inversus, this condition doesn’t usually have ill effects. It’s only when just some of the organs are reversed that there’s a potential for serious problems.
About 5 years ago, developmental biologists stumbled upon a potential explanation for the origin of a body’s normal left-right asymmetry. While studying mice, they found that an embryonic region called the node has hairlike cilia that twirl in a clockwise direction. The researchers also reported that this action creates a leftward current within the fluid bathing the node. Soon after the cilia appear during embryonic development, certain genes turn on in either the left or right sides of the mouse embryo.
These findings led the scientists to speculate that the ciliary action leads to cell-secreted chemical signals becoming concentrated on one side of the embryo and switching on genes there (SN: 8/21/99, p. 124).
Although virtually all scientists agree that this explanation for left-right asymmetry is elegant, some refuse to accept it.
“It’s a very appealing model, but I don’t think it’s consistent with the facts,” says Michael Levin of the Forsyth Institute in Boston.
On the basis of his work with frog and chick embryos over the past few years, Levin in an upcoming Bioessays makes a case against the cilia model and puts forth his own theory for how vertebrate embryos establish their two sides. The break in symmetry happens long before the cilia appear on nodal cells, asserts Levin. He argues that an asymmetric distribution of ions arises as early as the first few cell divisions of a vertebrate embryo. The uneven distribution of these charged atoms creates an electric field that pulls other ions and charged molecules to one side of the embryo or the other. This, Levin theorizes, ultimately triggers various genes to become active on only the right or left side of the embryo.
One-sided debate
For developmental biologists studying left-right asymmetry, the fundamental problem rests in the fact that the vertebrate embryo starts out as a seemingly uniform ball of cells. Through a variety of cues that scientists are still teasing out–gravity, the site of sperm entry to the egg, and the activity of maternal proteins stored in the egg–vertebrate embryos seem to establish top and bottom, as well as front and back, almost immediately after fertilization. Yet scientists used to think that the left-right distinction doesn’t arise until much later in the growth of an embryo, when organs start to take shape.
Over the past decade, biologists have documented several genes that have a left- or right-sided nature to their activity in the growing embryo. Perhaps the best-known one is nodal, named for the embryonic node. In all species examined so far, which include mouse, frog, and chick, nodal turns on initially in what will become the left side of an embryo. This gene appears to set off a cascade of asymmetric gene activity at about the time when the heart, intestines, and other internal organs begin to form.
Cilia entered the asymmetry story when researchers found that mutant mice without cilia in the node or with paralyzed cilia develop situs inversus or at least have some organs out of place. Biologists have even shown that artificially reversing the direction of the fluid flowing across the embryo’s node disrupts proper positioning of a mouse’s internal organs. The cilia must be pushing leftward signals that turn on nodal or other genes, many biologists assumed.
Yet no one has identified these signaling molecules, notes Levin. He and other investigators also question whether a cilia-driven current can consistently define left and right, given the intricate dynamics of fluid movement.
The most significant challenge to the hypothesis that cilia position organs, however, comes from the embryos of other vertebrates, such as frogs and chicks. In these animals, researchers have found genes and proteins with an asymmetric distribution of activity long before cilia appear in the embryonic node. For example, in the Dec. 27, 2002 Cell, Joseph Yost of the University of Utah in Salt Lake City and his colleagues reported that an enzyme that alters a protein called syndecan-2 is active only on the right side of an early frog embryo.
“The cilia are there in the frog, but they [appear] later than these left-right asymmetries,” says Yost.
Mind the gap
While working in the laboratory of Cliff Tabin at Harvard Medical School in Boston in the mid-1990s, Levin also documented one-sided gene activity in the chick before the cilia appear.
When he teamed up with Mark Mercola, then also at Harvard Medical School, Levin began to get the first hints of an alternative means by which the embryo might define left and right. The two researchers discovered that cell-to-cell portals known as gap junctions are required for proper left-right patterning.
Adjoining cells use gap junctions to exchange small molecules directly. Levin compares gap junctions to the hatches that link compartments in a submarine.
In experiments on early frog and chick embryos, he and Mercola disrupted gap junctions, either by applying drugs that block the portals or by mutating genes encoding the proteins that make up the junctions. These manipulations perturbed the cascade of asymmetric gene activity and caused the embryos to position some of or all their internal organs on the wrong sides.
But what’s flowing through the gap junctions that’s so important to left-right organ placement? And how do the portals create embryonic asymmetry, given that molecules can usually flow through them in both directions? Aware of the tendency for cells to transfer ions via gap junctions, Levin, Mercola, and their colleagues exposed early frog embryos to the hundreds of compounds known to affect ion movement into and out of cells.
The scientists then examined the treated embryos for left-right organ abnormalities.
Levin recalls that most other scientists thought this strategy was “insane” because they assumed that ion flow is so important to general development that the experimental embryos would simply die. Yet many embryos did survive, including some that had problems such as a heart looping the wrong way or a gallbladder on the left side.
Levin’s team discovered that the few drugs with such effects target select proteins that regulate the flow of potassium and hydrogen ions into and out of cells. Consider the drug lansoprazole, which prevents a cell-membrane protein called H+/K+-ATPase from swapping potassium ions outside a cell for hydrogen ions inside. More than half of the frog embryos treated with lansoprazole early in their growth develop left-right patterning defects, Levin, Mercola, and their colleagues reported in the Oct. 4, 2002 Cell.
Following up on that clue, the researchers examined the distribution of the H+/K+-ATPase within the early frog embryo.
Vertebrate embryos don’t immediately turn on their own genes but initially depend on the egg’s residual proteins, amino acids, and messenger RNA (mRNA), the instructions that a cell uses to make a protein. The scientists found that the fertilized frog egg starts with a symmetric distribution of mRNA for H+/K+-ATPase. But within the first or second cell division, this mRNA concentrates on the future right side of the embryo. This is the earliest left-right asymmetry that biologists have seen, and it occurs more than a day before cilia appear in the frog embryo.
“One of the big arguments in the field is, ‘When does the embryo know left from right?’ The frog embryo knows its left and right at the four-cell stage,” concludes Levin.
Levin, Mercola, and their colleagues also found that chick embryos early on, before they have cilia, develop a voltage between what will become their left and right sides. This electric asymmetry appears to arise because the cells on one side of the embryo use ion channels and pumps to drive positively charged ions, such as potassium, out of the embryo. Ultimately, the cells on that side have a more negative charge than do those on the other side.
Levin suggests that the natural electric field established by the asymmetric ion flux pulls charged signaling molecules through gap junctions in a directed manner, concentrating the signals on one side of the embryo or the other. Those signals, in turn, could set off the left- and right-sided cascades of gene activity that guide the growth and positioning of organs.
The electric field could operate on ions such as hydrogen or calcium. Mice genetically engineered to lack polycystin-2, a protein that controls the release of calcium ions within cells, show disturbed left-right patterning, notes Levin.
Another possible signal is serotonin, a chemical best known as a transmitter of nerve signals. At this summer’s Society for Developmental Biology meeting in Boston, Levin’s colleague Takahiro Fukomoto reported that frog and chick embryos treated with drugs affecting serotonin’s activity show disrupted left-right asymmetry. Since it’s a relatively small, charged molecule, serotonin is an “ideal candidate” for one of the left-right patterning signals that flow through gap junctions, says Levin.
Electrifying appeal
Levin acknowledges that his theory doesn’t explain how normal left-right asymmetry gets started: Something has to first distribute H+/K+-ATPase and other ion pumps and channels unevenly. The experiments on frog embryos indicate that this biased allocation begins almost immediately after fertilization, so the left-right axis is probably established at about the same time as are the front-back and top-bottom axes.
“We still don’t know step one of [left-right] asymmetry,” says Levin. “We now have it trapped in an hour-and-a-half slot.”
Levin’s theory has won the support of some developmental biologists, especially those skeptical of the role of cilia. “I love it,” says Lewis Wolpert of University College London. “I can’t say whether it’s right or wrong, but I find it terribly interesting.”
Wolpert also appreciates that Levin’s work has brought renewed attention to natural electric fields within organisms.
This concept has been around for decades, according to Kenneth R. Robinson of Purdue University in West Lafayette, Ind., who collaborated with Levin and Mercola on the recent studies. In the August Bioessays, he and a colleague review the growing evidence that endogenous electric fields have roles in embryonic patterning, wound repair, tissue regeneration, and plant biology.
Robinson chides other molecular biologists for ignoring endogenous electric fields in favor of genes and biochemistry.” They basically just don’t know what an electrical field is. When you talk about these issues, a lot people just don’t get it because they’re not intellectually prepared,” he says.
While calling Levin’s work innovative, Tabin still supports a cilia-based mechanism for left-right determination. In the Jan. 1 Genes and Development, he and Kyle J. Vogan of Harvard Medical School tried to reconcile some of the field’s conflicting data by proposing that the node contains two kinds of cilia: A twirling set establishes a leftward flow of fluid, and immobile cilia respond to this flow by releasing intracellular calcium and activating genes such as nodal.
In the July 11 Cell, a research group led by Martina Brueckner of Yale University School of Medicine confirms the presence of these two types of cilia in the mouse node and reports a surge of calcium into cells on the left side of the node at the same time that the twirling cilia establish a leftward flow of fluid.
Gap junctions could propagate such a calcium surge through other parts of the embryo, notes Mercola. Despite working with Levin, he still thinks that cilia may represent the first step in left-right determination in many vertebrates.
“Maybe chicks and frogs initiate asymmetry with a different mechanism,” he says. “I’d caution against the idea that there’s one way to bootstrap left-right asymmetry and argue instead that the jury is still out. It might indeed turn out that nature has employed multiple means in different species to initiate left-right asymmetry.”
Yost points out that part of the field’s disagreement arises because scientists working on different animals have focused on different stages of embryonic development. He predicts that investigators will find left- or right-sided gene activity in mouse embryos before cilia appear.
Yet Levin’s results don’t necessarily eliminate a role for cilia, Yost stresses. The cilia could lock in any left-right decision that the vertebrate embryo has made earlier or spread its effects beyond the node, he points out.
“I think the two models are not mutually exclusive at this point,” Yost says. “My sense right now is that asymmetry will be set up in the same way in all vertebrates. We just don’t know how that happens at this point.”
****************
If you have a comment on this article that you would like considered for publication in Science News, send it to editors@sciencenews.org. Please include your name and location.