Smuggling a CRISPR gene editor into staph bacteria can kill the pathogen
The technique takes advantage of the way the microbes naturally swap genes to become more harmful
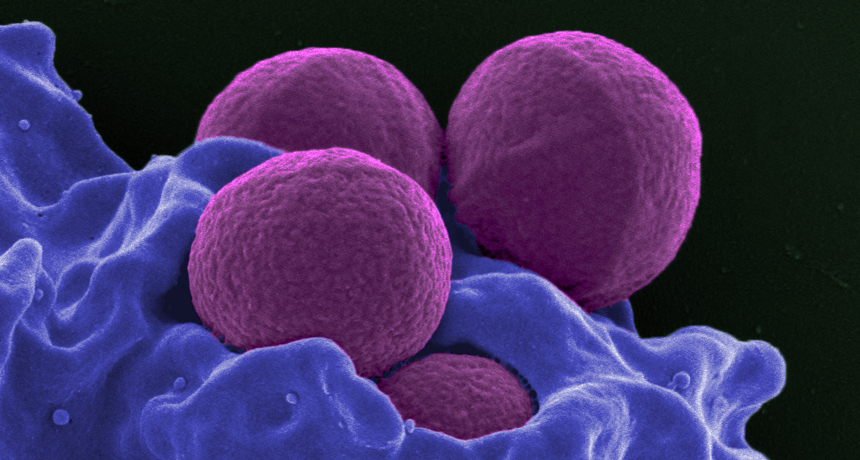
BATTLING A SUPERBUG A new approach to fighting antibiotic-resistance Staphylococcus aureus bacteria (purple spheres, shown in this scanning electron microscope image being ingested by a white blood cell) co-opts genes that normally make the bacteria more dangerous.
NIAID/Flickr (CC BY 2.0)