How to make CAR-T cell therapies for cancer safer and more effective
Researchers are installing safety switches and adding other features to the immune therapy

CAR-T 2.0 An immune therapy for cancer using CAR-T cells (two shown in yellow, attacking a breast cancer cell) made a big splash in 2017, but it comes with serious side effects that researchers are trying to combat.
Eye of Science/Science Source
This wasn’t 15-year-old Connor McMahon’s first time in the hospital. But the 107° fever he’d been running for three days had his dad frightened. The teen was hallucinating, talking gibberish and spouting curses.
“I thought he was going to die,” says Connor’s father, Don McMahon, who stayed close as his son received and recovered from an experimental treatment for leukemia. “It was really hard to watch.” But the fever finally broke, and Connor returned home. Just a month later, in November 2016, he was cancer-free and back on the ice in his hockey skates and pads.
That episode was Connor’s third bout with acute lymphoblastic leukemia. The experimental treatment was a last hope for the boy, who was first diagnosed at age 3. He has spent a total of six years of his life receiving chemotherapy. When the cancer came back in 2016, the doctors said the prognosis wasn’t good.
At that point, “it was about quality of life, not quantity,” McMahon says. But when he and his wife, Michelle, learned about the experimental treatment, called CAR-T cell therapy, the family decided it wasn’t time to give up yet.

A month later, doctors injected those modified T cells into the teen’s body, where they multiplied. Over the next few weeks, that five-minute T cell infusion racked Connor’s body but also knocked the levels of cancer cells in his bloodstream down to zero.
CAR-T cell therapy has captivated cancer researchers and patients alike because of stories like Connor’s. The U.S. Food and Drug Administration approved two versions in late 2017. In clinical trials, the treatment that Connor took, called Kymriah, wiped away all signs of acute lymphoblastic leukemia in 52 of 68 children and young adults. Of those, 75 percent were still cancer-free six months later. And in a study of 101 patients taking Yescarta, a CAR-T therapy for adults with certain types of lymphoma, 51 percent of patients showed no sign of cancer after treatment. (In May, the FDA also approved Kymriah for people with certain lymphomas.) These results were particularly exciting because the patients in these trials were dealing with a recurrence of cancer or had been through at least two other treatments that didn’t work.
But these new therapies can come with scary side effects. A majority of patients who receive CAR-T cell therapy react like Connor did, with varying degrees of severity. Those same T cells that are outfitted to attack the cancer can send the immune system into overdrive by instigating a surge of proteins called cytokines into the bloodstream, triggering inflammation. Cytokine release syndrome, as it’s called, can cause high fevers, make patients’ hearts race out of control and send blood pressure plummeting. The cytokines can also attack the brain, causing seizures.
In a large, multihospital study of Kymriah, 54 of 68 patients experienced some form of cytokine release syndrome. Symptoms were severe enough in 32 patients to require intensive care hospitalization. Neurological problems are a risk as well. In 2016, five patients died from fatal brain swelling in a CAR-T cell clinical trial run by Seattle-based Juno Therapeutics. The company stopped the trial, and the deaths intensified questions about the treatment’s safety.
These side effects are one reason why both therapies are approved for only a narrow range of patients: people with a few very select kinds of blood cancers — certain types of leukemia and lymphoma — and only those whose cancer hasn’t responded to conventional treatments. And CAR-T therapy is offered only at select cancer treatment centers with trained teams that follow rigorous safety protocols to control side effects. Yescarta, for example, is available at just over 50 U.S. hospitals.
Many oncologists are convinced, however, that someday CAR-T cells could be used on a much wider group of patients and for a broader spectrum of cancers. Researchers are designing new forms of the treatment, changing the way that patients’ T cells are engineered to make the therapy less risky. Scientists are installing safety switches that can turn off CAR-T cells on command and designing T cells that activate only under certain conditions. Others are adding features that help the T cells more specifically target cancer cells but ignore healthy cells, or reduce collateral damage from T cell–boosting drugs. Meanwhile, doctors are learning how to better manage the side effects for patients.
“I think this is an incredibly exciting time,” says David Maloney, a physician and CAR-T cell researcher at the Fred Hutchinson Cancer Research Center in Seattle. The CAR-T cell technology is rapidly improving, and “our job is to do this safely and make it even more effective.”
Building a better T cell
Some immune system cells recognize cancer cells as abnormal and kill them, but sometimes that’s not enough. Researchers have tried for decades to harness the immune system to fight cancer more effectively. Doctors have used vaccines or given patients lab-made versions of immune system proteins to heighten patients’ immune responses.
“Immunotherapy goes back a long time, but successful immunotherapy is just in its infancy,” says Paul Martin, a pediatric oncologist at Duke University who treated Connor with CAR-T cells. He says CAR-T therapy is part of a new, more promising wave of immune therapies against cancer.
The underpinnings of CAR-T cell therapy were developed in research labs in the 1990s, and the first CAR-T cells fought off cancers in mice in the early 2000s. But with last year’s FDA approvals for the two CAR-T cell therapies, interest has exploded (SN: 12/23/17, p. 29). As of June 14, the government’s registry of clinical studies, ClinicalTrials.gov, included 272 active studies for CAR-T cell therapies, mostly in the United States, China and Europe. Hundreds more variations are being designed and tinkered with in research labs around the world.
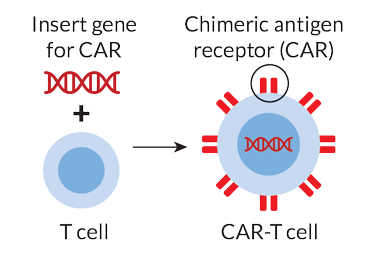
CAR-T cell therapy works by taking advantage of cells’ natural communication systems. The surface of every cell is studded with proteins that send messages between cells in a sort of molecular semaphore. The engineered T cells have a chimeric antigen receptor, or CAR, on their surface — a synthetic protein that has a laser-focus lookout for a specific protein that appears on the surface of certain cancer cells. Most CAR-T cells developed to date are aimed at a molecule called CD19 found on the surface of B cells, another kind of immune cell that, when it multiplies unchecked, leads to certain blood cancers.
So far, CAR-T cell therapy has been approved only for certain leukemias and lymphomas that start in B cells. Those cancers are among the easiest to develop such a treatment for, Martin says. “This was the low-hanging fruit.” Blood cancers are easier to root out than solid tumors, which have hard-to-reach cells buried within and develop a fortress of defense against cancer therapies. A handful of CAR-T therapies aimed at solid tumors are in early patient studies.
Hitting the bull’s-eye
Right now, the CAR-T cells’ aim isn’t perfect — they often hit healthy B cells that also display the protein the T cells are targeting, sending ripple effects through a patient’s immune system. For CAR-T therapy to be anything other than a treatment of last resort, scientists will need a better level of control over the cells than exists now, says Kole Roybal, an immunologist at the University of California, San Francisco.
Roybal is working on a system to control when and where in the body engineered T cells are activated. It adds an extra step to turning on the T cells, like a Breathalyzer test that a driver must pass before a car can be started.
The system relies on a synNotch receptor — a second synthetic protein that scientists can add to the surface of T cells in addition to a CAR. Just like a CAR, the synNotch receptor recognizes a specific protein on a cancer cell’s surface, and then latches on.
But synNotch, which hasn’t been tested in patients yet, doesn’t trigger the T cell to kill the cancer cell. Instead, when synNotch sees a cancer cell, it directs the T cell to make a CAR appear on the cell surface. That way, the only T cells with killing potential are the ones that are in the presence of cancer cells, Roybal says. His team reported the development of CAR-T cells with synNotch receptors in February 2016 in Cell. In tests in human cells and in mice, T cells with synNotch receptors targeted cancer cells more selectively than CAR-T cells without the synNotch receptors.
Other scientists are working on a different problem: how to turn CAR-T cells off once they’ve done their job. That’s important to do because Connor and patients like him will have a small number of CAR-T cells hanging out in their bloodstream for their entire lives. Those cells will kill healthy B cells, weakening the body’s ability to fight off ordinary illnesses. To counteract the effect, Connor needs years of weekly infusions of a protein called immunoglobulin, his father says.
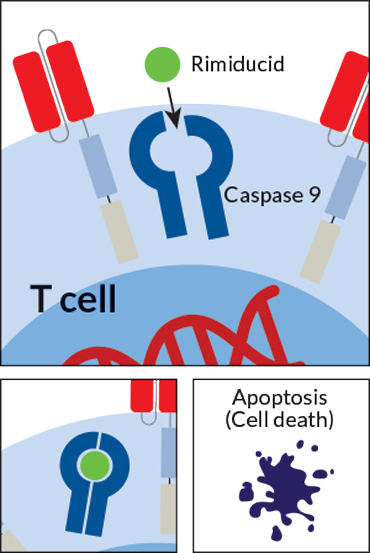
So scientists are building several versions of CAR-T cells with an “off” switch. The cells are designed to self-destruct when they encounter a particular synthetic small molecule or protein that is not normally found in the body. The patient could take the small molecule as a drug when it’s time to turn off the T cells. The drug-induced off switch could be used to flush CAR-T cells out of a patient’s system once the treatment has run its course, or to disable the T cells if a patient starts experiencing dangerous side effects.
Some of these switches are already being tested in patients. One switch, designed by Houston-based Bellicum Pharmaceuticals, responds to the drug rimiducid. A CAR-T cell therapy with this switch is in early testing in children, says Bellicum spokesperson Brad Miles.
Other, more sophisticated versions are being developed in the lab as well. For example, the pharmaceutical company Cellectis, based in New York and Paris, is testing an all-in-one safety CAR; the self-destruct switch is part of the CAR itself rather than a separate apparatus on the cell. Integrating the two is a tougher engineering challenge than adding a separate safety switch, says Julien Valton, a Cellectis researcher. In lab tests of the all-in-one prototype, it takes about 10 minutes to shut off the cells if they’re in a petri dish, Valton says, but about four days for cells circulating in the bloodstream of a mouse.
Safer cytokines
There may be a more targeted way to amplify the power of the engineered T cells within a patient.
For instance, the cytokine interleukin-2 is a master driver of the immune system. Normally, IL-2 is present in small quantities, and can boost the size of the body’s T cell population.
In theory, giving IL-2 to patients on CAR-T cell therapy could be a way to make the modified T cells mount a stronger response against the cancer. But it also makes regular, nonengineered T cells multiply. That’s risky: The influx of T cells quickly sends the immune system into hyperdrive, making people very sick. “It sounds good on paper, but in practice, it’s not what these molecules were evolved to do,” says Jonathan Sockolosky, who modified IL-2 while at Stanford University. He has since moved on to other projects at South San Francisco–based Denali Therapeutics.
By tweaking the way IL-2 interacts with the immune system, Sockolosky and his team found a way to make CAR-T treatment more targeted, they reported in Science in March. With the chemical modifications, the IL-2 communicates with and boosts production of only the modified T cells designed to fight cancer, not the rest of the body’s T cells. In tests in mice, the IL-2 that enhanced the CAR-T cell activity barely bound to the body’s normal T cells at all.
A universal CAR-T cell
Making CAR-T cells better isn’t all about making them more specific, though. A big limitation of today’s CAR-T cell therapies is that they must be personalized for each patient, which makes them time- and cost-intensive to produce. Right now, one dose of Kymriah, which is usually all a patient needs, costs $475,000.
Some scientists are working on a universal CAR-T cell therapy — one that doesn’t rely on cooking up small batches of personalized T cells for each patient. Hospitals could carry a stock of CAR-T therapies much like a stash of antibiotics or pain meds. Moving away from the made-to-order treatments could make it easier for smaller cancer centers to provide the therapy, increasing access for patients. It could also shorten the wait time for patients to be treated, since cells wouldn’t need to be shipped to a lab to be modified and duplicated.
From a drug company’s perspective, it’s advantageous to develop a single uniform product rather than to market a procedure, which is essentially what other available CAR-T cell therapies are, says André Choulika, CEO and cofounder of Cellectis.
The universal approach would work much like today’s CAR-T cell therapies, Choulika says. But it would use T cells from a healthy donor instead of the patient with cancer. So a lab could make many doses of engineered T cells from the same starting cells. The cells would be edited to remove the genetic instructions that normally help T cells distinguish foreign cells from the body’s own cells. That’s an important step, because normally a patient’s immune system won’t accept an infusion of T cells from someone else’s immune system.
Universal CAR-T cells are already being tested in people, but the road has not been smooth: In 2017, Cellectis was forced to temporarily halt clinical trials for one of its products after a patient died from severe cytokine release syndrome. The trials have since restarted with a lower dose of CAR-T cells and tighter restrictions on who can participate. Choulika estimates it will be three to four years before any of the company’s universal CAR-T treatments make it through the clinical trials process.
Meanwhile, Boston University biomedical engineer Wilson Wong is trying to address the smorgasbord of challenges presented by CAR-T therapies in one streamlined system — something like the Swiss army knife of CAR-T cells, he says. His design splits up the CAR into multiple pieces, forcing the T cells to recognize two different proteins on the cancer cell’s surface to kill it. That helps reduce the off-target effects.
And the system is adaptable on the fly. Right now, Wong says, CAR-T cells are engineered to have a specific protein on the outside — one that recognizes a certain matching protein on the cancer cell’s surface. But not all kinds of cancer cells have a protein that’s specific only to the cancer and not also found on healthy cells. And sometimes cancer cells mutate and change the proteins they show on their surfaces, allowing the cancer to hide from CAR-T cells.
In Wong’s system, the cancer cell–sensing proteins aren’t built directly into the CAR-T cell. Instead, they’re attached to a drug that’s engineered to stick to the T cell. When the drug is injected into a patient’s bloodstream, it tracks down and attaches to the CAR-T cells, only then giving those engineered cells the ability to hunt and kill cancer cells.

CAR-T cell futures
It’s far too soon to say that Connor has been cured of cancer. But he’s been cancer-free for more than 18 months — an outcome that in 2016, he and his family couldn’t have imagined. He just dyed his hair blond. In March, his hockey team took second place in its division at the USA Hockey Youth Nationals in Wayne, N.J.
Don McMahon understands why, right now, CAR-T cell therapy is so limited. But he also marvels at his family’s luck: Connor had exactly the right kind of cancer to receive the treatment, and he met the stringent criteria to participate in the clinical trial. Other patients might not be so lucky. “It seems so arbitrary,” he says, while acknowledging that the restrictions are in place for good reasons. He hopes that, as research progresses, CAR-T cell therapy can help more patients.
As CAR-T therapy improves, it may do what McMahon hopes. “We’ll still need chemo and radiation and surgery,” says Martin, Connor’s oncologist, “but now we’re going to have a whole new addition — immunotherapy.”
This article appears in the July 7, 2018 issue of Science News with the headline, “CAR-T 2.0: Can the risks of this promising immune therapy for cancer be tamed?”