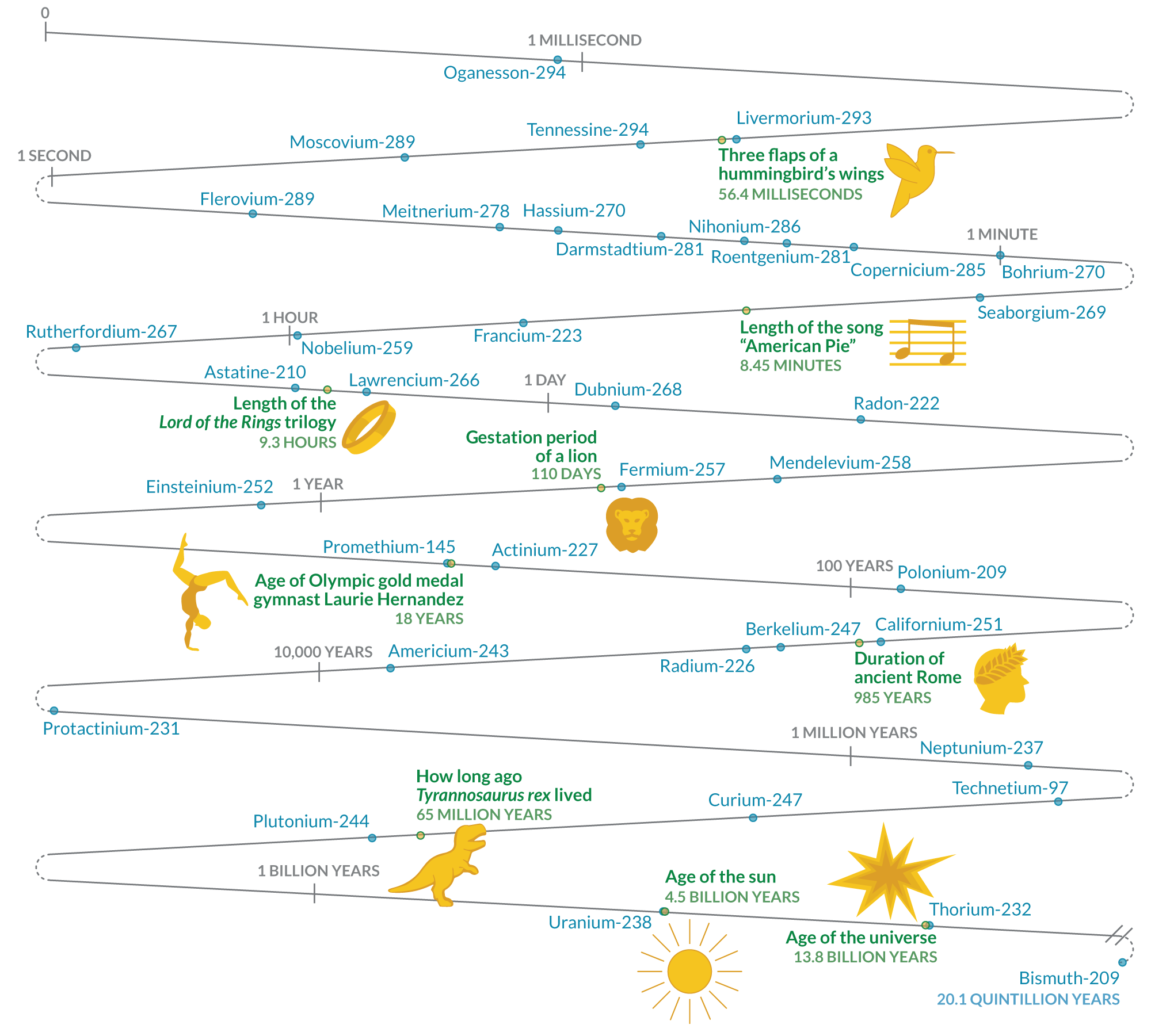
Here’s how long the periodic table’s unstable elements last
Some of these isotopes have half-lives of seconds or less, while others last and last

HALF-LIFE COUNTDOWN Generally, for elements after uranium on the periodic table, the further along elements are — the higher their atomic number — the less time they endure.
KamilSD/Alamy Stock Photo